T-cell immunoglobulin and mucin domain-containing protein 3 (TIM3), also known as HAVCR2, is a member of the TIM family. It participates in regulating both innate and adaptive immune responses and generally has inhibitory functions. By binding to its ligand, TIM3 inhibits T cell activation and proliferation, reducing the secretion of cytokines such as IFN-γ and TNF-α. TIM3 is highly expressed in tumor-infiltrating lymphocytes in various cancers and is closely associated with tumor progression and immune escape, playing a crucial role in tumor immune responses.
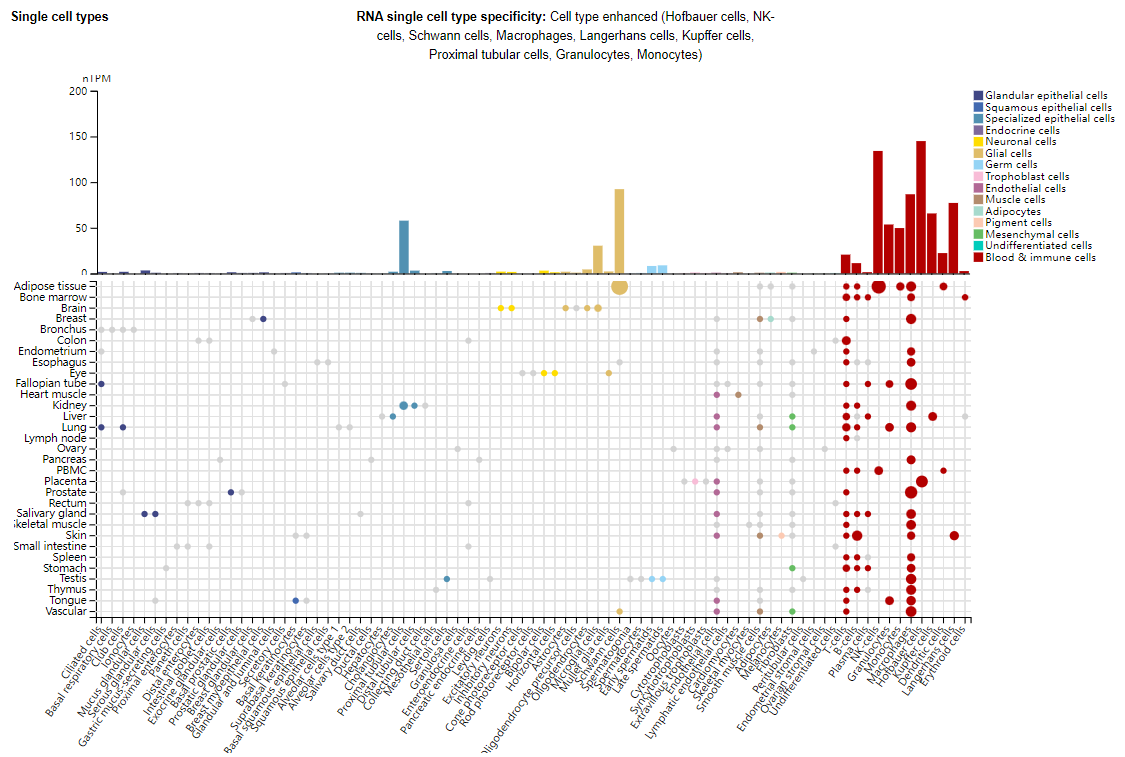
TIM3 expression distribution
TIM3 is expressed on a variety of immune cells, including CD4 + T cell subsets (Th1/Th17 cells), regulatory T cells (Tregs), CD8 + T cell subsets, and innate immune cells (such as dendritic cells, NK cells, monocytes, and macrophages). In addition, it is also expressed in glial cells and specialized epithelial cells.
(Data source: Uniprot)
Structure of TIM3 and its ligands
TIM3, encoded by Havcr2, is a type I membrane protein composed of 301 amino acids, a variable Ig domain (IgV), a glycosylated mucin domain of variable length, a single transmembrane domain, and a C-terminal cytoplasmic tail containing a conserved tyrosine-based signaling motif. Unlike other checkpoint receptors PD-1 and TIGIT, a unique feature of TIM3 is the lack of known inhibitory signaling motifs in its cytoplasmic tail. TIM3 has four ligands: galectin-9, phosphatidylserine (PtdSer), high-mobility group box 1 protein (HMGB1), and CEACAM-1.

(Data source: Kandel S, Adhikary P, Li G, Cheng K. Cancer Lett. 2021)
TIM3 signaling pathway and regulation:
In T cells, in the absence of TIM3 ligands, Bat-3 binds to the cytoplasmic tail of TIM3 and associates with the catalytically active Lck. Lck then phosphorylates the CD3ζ subunit of the T cell receptor (TCR) complex, subsequently recruiting the ζ chain-associated protein kinase (ZAP70) to the TCR complex. This recruitment leads to activation of the ZAP70/T cell activating ligand (LAT)/phospholipase Cγ1 (PLCγ1)/Ca2 + pathway to promote T cell proliferation and survival. However, ligand ligation of TIM3 displaces Bat-3 from the TIM3 tail, leading to the recruitment of tyrosine phosphatases (CD45 and CD148), resulting in the dephosphorylation (inactivation) of Lck, downregulating the ZAP70/LAT/PLCγ1/Ca2 + TCR signaling pathway and inhibiting T cell proliferation and survival.

In DCs, TIM3 inhibits HMGB1 expression, thereby suppressing NF-κB-mediated DC activation. TIM3 binds to DCs and activates Btk and c-Src, thereby inhibiting NF-κB activation. TIM3-mediated DC inhibition can inhibit CXCL9 production, thereby reducing the recruitment of CD8+ T cells to the TME.

(Data source: Acharya N, et al. J Immunother Cancer. 2020)
TIM3 targeted therapy
Currently, the main treatments targeting TIM3 are monoclonal antibodies and bispecific antibodies, which can improve anti-tumor immunity, especially when used in combination with PD-1 blockade, which supports the development of TIM3 as an immunotherapy target.
Sabatolimab, a TIM3-targeting monoclonal antibody developed by Novartis, is currently in Phase 3 clinical trials for the treatment of chronic myelomonocytic leukemia, myelodysplastic syndrome, and acute myeloid leukemia. In December 2024, Novartis announced a clinical trial (NCT05201066) aimed at completing a previously sponsored study of sabatolimab (MBG453) and conducting a rollout study in patients who, in the opinion of the investigators, benefited from continued sabatolimab treatment.
Cobolimab (TSR-022) is a humanized anti-TIM-3 IgG4 antibody developed by Tesaro (acquired by GSK). It is currently in Phase 3 clinical trials for the treatment of advanced non-small cell lung cancer, advanced cervical cancer, and cervical metastatic cancer.
Lomvastomig is a bispecific antibody targeting PD-1 and TIM-3. By simultaneously targeting two immune checkpoint proteins, PD-1 and TIM-3, Lomvastomig enhances the immune system's attack on tumor cells. It is primarily used to treat advanced or metastatic esophageal squamous cell carcinoma, metastatic melanoma, non-small cell lung cancer, and small cell lung cancer. A Phase II randomized, blinded, active-controlled, global multicenter study was planned to evaluate the safety and efficacy of lomvastomig compared with nivolumab in patients with advanced or metastatic esophageal squamous cell carcinoma (ESCC). However, due to strategic considerations, recruitment of the lomvastomig group was stopped in June 2021. Lomvastomig is currently in a Phase I clinical trial for metastatic melanoma. According to GlobalData, Phase I drugs for metastatic melanoma have a Phase Transition Success Rate (PTSR) benchmark of 81%, which allows them to enter Phase II clinical trials.
Sabestomig is a bispecific antibody developed by AstraZeneca and is currently in Phase 1/2 clinical trials for the treatment of advanced malignant solid tumors, gastroesophageal junction cancer, and metastatic solid tumors.
