TL1A is a member of the tumor necrosis factor ligand superfamily, member 15 (TNFSF15), also known as TL1 and VEGI. DcR3 is a functional ligand for TL1A, which plays an important role in regulating innate and adaptive immunity. It is a therapeutic target for inflammatory bowel disease (IBD), allergic diseases, and autoimmune diseases.
Expression distribution of TL1A
TL1A is mainly expressed in glandular epithelial cells, squamous epithelial cells, specialized epithelial cells, and also in immune cells such as macrophages, dendritic cells, and Langerhans cells.
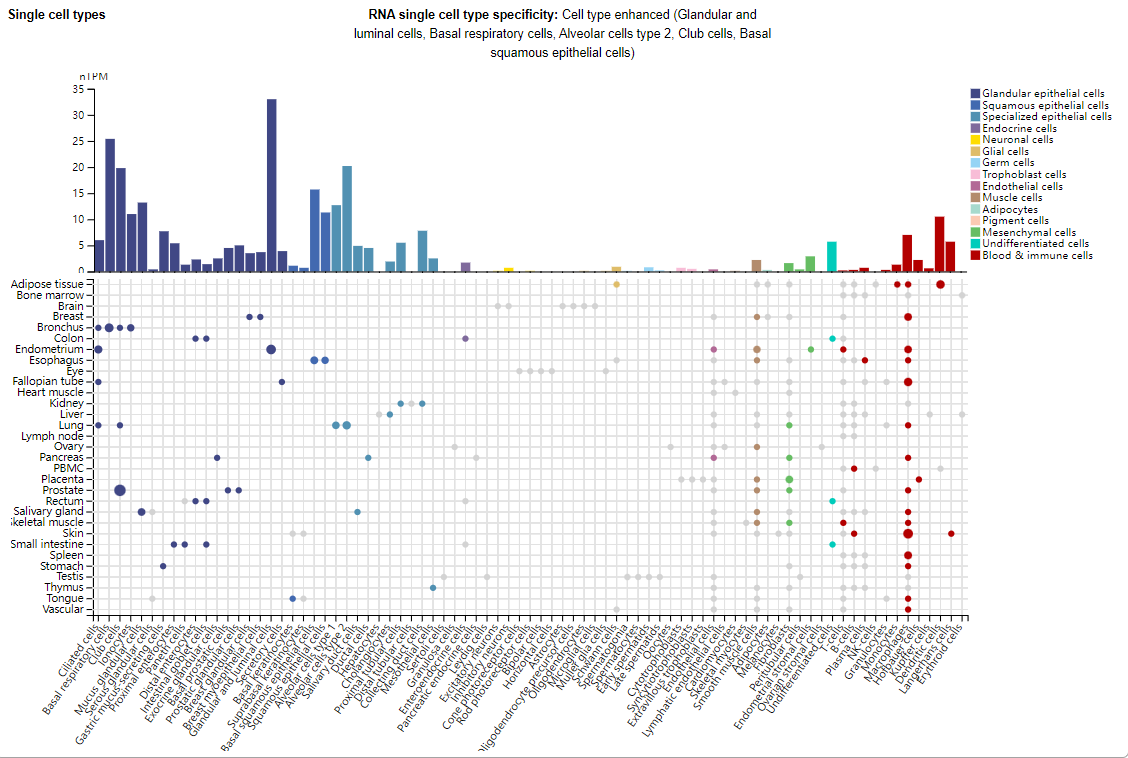
(Data source: Uniprot)
Structure of TL1A and its receptor
TL1A is a type II transmembrane protein consisting of cytoplasmic, transmembrane, and extracellular domains. The C-terminal extracellular domain contains the typical TNF homology domain (THD), composed of antiparallel β-pleated sheets, forming a trimer structure. Like other TNF family members, TL1A exists on the cell surface as a stable trimer and a membrane-bound (mTL1A) isoform. sTL1A is a soluble, fully functional isoform of mTL1A generated by matrix metalloproteinase (TACE). The functional receptor for TL1A is DR3, a member of the TNFR superfamily (TNFRSF25). DR3 is a type I protein with extracellular, transmembrane, and cytoplasmic domains. TL1A binds to DR3 trimers as a trimer, forming a 3:3 complex.

(Data from Zhan C, Patskovsky Y, Yan Q, et al. Structure. 2011)
TL1A signaling pathway and regulation:
The intracellular region of DR3 contains a characteristic configuration of a death domain that signals through TRADD. Upon binding to TL1A, downstream signaling may take one of two distinct pathways, depending on the cellular and molecular context of ligand binding. In the first scenario, TL1A binding to DR3 may activate signaling pathways such as NF-κB, MAPK, and PI3K, promoting inflammatory responses. In the second scenario, TRADD dissociates from the DR3 death domain and binds to FADD in the cytoplasm, leading to the activation of caspase-8 and caspase-3. The result in this scenario is programmed cell death (PCD)-induced apoptosis.
TL1A can also bind to the decoy receptor DcR3 (TNFRSF6B), which competes with DR3 for ligand binding, thereby inhibiting the functional effects of TL1A.

(Data source: Bamias G, et al. Gut. 2024)
The role of TL1A in immune regulation
As a co-stimulatory molecule: TL1A:DR3 is a potent co-stimulatory system that effectively amplifies adaptive immune responses across various immunophenotypes.
Regulatory T cell subsets: including Th1, Th17 and Th9 cells, which can also activate regulatory T cells (Tregs) and play an important role in maintaining immune homeostasis.
Regulating innate immune response: TL1A can regulate the function of ILCs through the DR3 signaling pathway, promote the secretion of cytokines such as IL-22, and thus maintain the homeostasis of the intestinal mucosa.
Involved in the regulation of inflammatory responses and intestinal mucosal barrier function: promoting the recruitment and activation of inflammatory cells and maintaining intestinal mucosal integrity. Abnormal expression of TL1A is closely associated with the occurrence and progression of inflammatory bowel disease, allergic diseases, and autoimmune diseases.

(Data source: Bamias G, et al. Gut. 2024)
Involvement in fibrosis: TL1A plays a crucial role in intestinal fibrosis, promoting fibroblast proliferation and collagen synthesis through the DR3 signaling pathway, thereby exacerbating the fibrotic process. In IBD patients, elevated TL1A expression is closely associated with intestinal fibrosis.

(Data source: Bamias G, et al. Gut. 2024)
Targeted therapy for TL1A
Targeted therapies for TLIA currently primarily focus on monoclonal antibodies, with many currently in clinical development. Afimkibart (PF-06480605), a fully humanized IgG1 monoclonal antibody targeting TL1A, developed by Pfizer, is being evaluated in a Phase 2a study (NCT02840721 (TUSCANY)) evaluating the efficacy, safety, tolerability, and pharmacokinetics of PF-06480605 as induction therapy in 50 patients with moderate-to-severe UC. Patients received 500 mg of PF-06480605 intravenously every two weeks for seven doses and were followed for three months. The study revealed 18 treatment-related adverse events, the most common of which were UC exacerbation and arthralgia, and four serious adverse events. PF-06480605 was associated with statistically significant improvements in endoscopic and histological outcomes at week 14. PF-06480605 is currently undergoing a Phase 3 clinical trial.
A phase 3, randomized, double-blind, placebo-controlled, induction and maintenance study evaluating the efficacy and safety of PRA023 (now called Tulisokibart) in the treatment of moderately to severely active UC is currently underway (NCT06052059). Results of the study showed that it resulted in clinical remission at 12 weeks.
Duvakitug (TEV 48574) is a monoclonal antibody targeting TL1A developed by Watt Pharmaceuticals. The investigational drug TEV-48574 may have dual anti-fibrotic and anti-inflammatory effects and is currently undergoing a Phase 2 study in patients with ulcerative colitis (UC) and Crohn's disease (CD).

(Data source: Solitano V, et al. Med. 2024)
PF-07261271 is a bispecific antibody developed by Pfizer that targets TNFSF15 (TL1A) and the p40 subunit of IL-12 for the treatment of inflammatory bowel disease. It is currently in Phase 1 clinical trials.
Recently, the Clinical Trial Application (IND) for SSGJ-627 Injection, an anti-TL1A monoclonal antibody developed by Sunshine Guojian, has been officially accepted by the Center for Drug Evaluation (CDE) of the National Medical Products Administration. This marks the first step in clinical research on this target in China, making SSGJ-627 the first TL1A-targeted drug to receive an IND in China.
