Background
CD46 is a membrane cofactor protein (MCP) that originally regulates complement activation in host cells. It is expressed in four major isoforms in all nuclear cells and is also involved in the regulation of human reproduction, T cell activation, immune-inflammatory effector functions, autophagy, and the newly discovered intracellular complement system (complex). It helps mitigate complement system activation, thereby focusing and limiting complement attack on invading pathogens and damaged tissues. CD46 is also involved in proliferation and cellular metabolism and serves as a receptor or adhesion factor for at least 12 pathogens. CD46 has recently emerged as a key player in cancer biology. Numerous studies have demonstrated a correlation between elevated CD46 expression, malignant transformation, and metastatic potential. These characteristics, along with its role as a pathogen receptor, make CD46 a target for cancer therapy. Modified viral vectors targeting CD46 (such as adenovirus and measles virus) are currently being used to treat a variety of cancers. Another cancer therapy utilizes human monoclonal antibodies targeting CD46 as antibody-drug conjugates.

In 2025, Liszewski MK and Atkinson JP from the Department of Medicine at Washington University School of Medicine published an article titled “ The multiverse of CD46 and oncologic interactions ” in J Clin Invest, which reviewed the “multiverse” of CD46 and its interactions with cancer.
Expression and structure of CD46
CD46 was originally discovered during the search for complement receptors and regulatory proteins that bind to C3b and C4b . The CD46 gene is located on 1q32 and comprises 14 exons and 13 introns , with a minimum length of approximately 43 kilobases . In addition to red blood cells, CD46 is expressed as a family of four major isoforms in varying proportions on most cells.
(Data source: Uniprot)
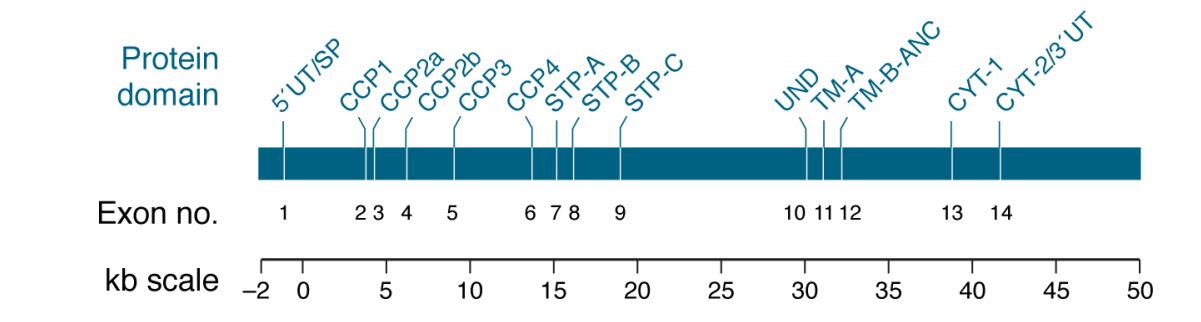
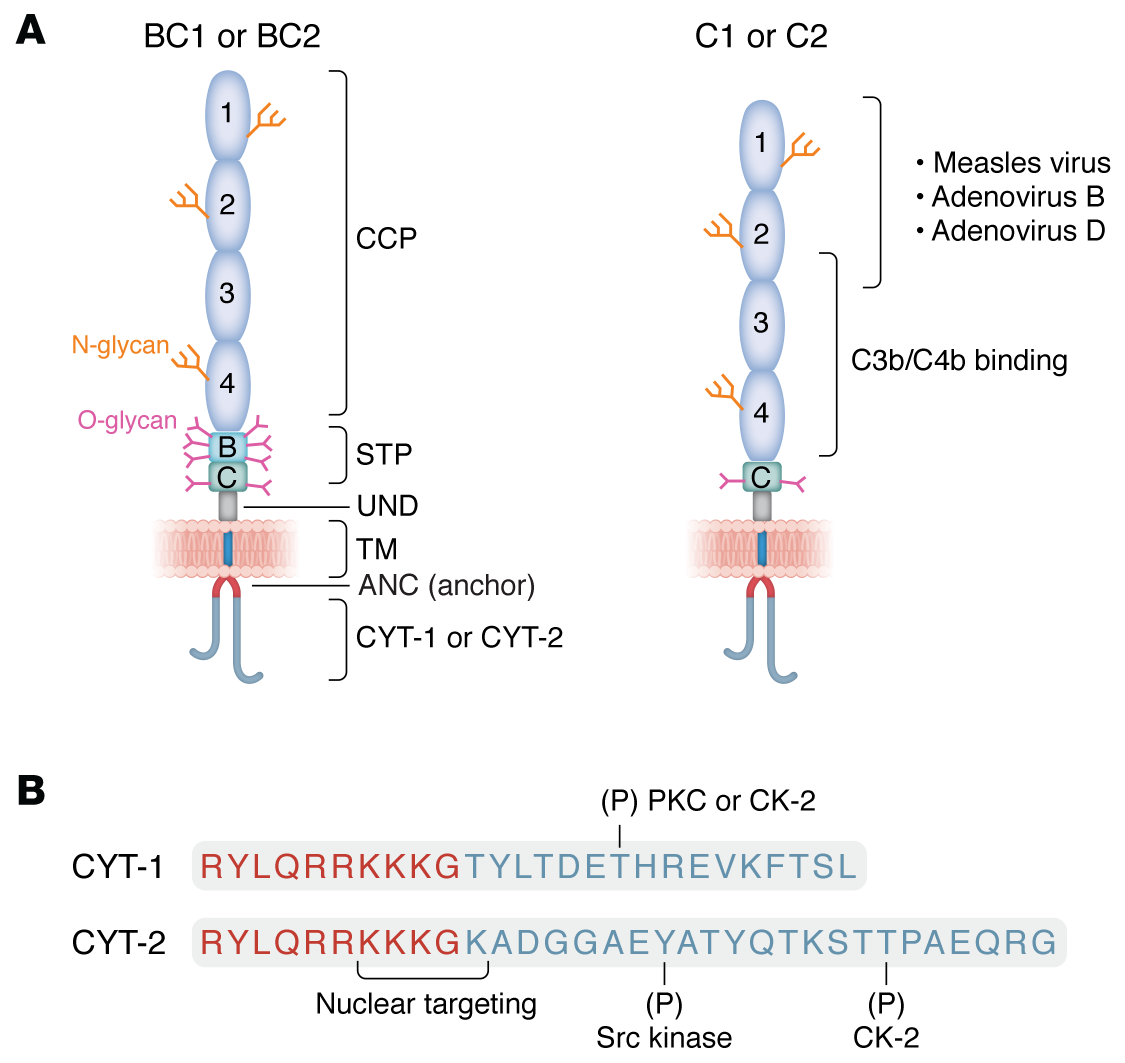
The structure of CD46 is primarily composed of four CCP repeats of approximately 60 amino acids each. CCP2-CCP4 are the primary sites of C3b/C4b regulatory function. CCP1, CCP2, and CCP4 possess N-glycosylation sites. Alternatively spliced STP fragments are also present, which serve as O-glycosylation sites. This is followed by a short segment (13 amino acids) of undefined function (UND) and a transmembrane (TM) and intracellular anchoring structure. Alternative splicing also generates two separate cytoplasmic tails (CYT-1 and CYT-2) with distinct signaling motifs. Potential intracellular phosphorylation and nuclear localization signal sites exist. Most cells express four common CD46 isoforms, designated BC1, BC2, C1, and C2.
Differences in glycosylation of the STP domain largely explain its broad or dual-band electrophoretic profile; high-molecular-weight species contain BC1 and/or BC2 isoforms, whereas less glycosylated low-molecular-weight species contain C1 and/or C2 isoforms.
Function of CD46
CD46 is a regulator of complement activation. The BC isoform has a stronger protective effect against the classical pathway than the C isoform. CD46 has a particularly strong inhibitory effect on activation of the self-amplifying alternative pathway. The two cytoplasmic tails of CD46 differentially mediate intracellular signaling that influences cell behavior. In epithelial cells, CD46 regulates autophagy upon pathogen invasion. This is mediated by CYT-1, which connects to the autophagosome through interaction of its C-terminal tetrapeptide (FTSL) with the scaffolding protein GOPC. CYT-1 also binds to the scaffolding protein DLG, mediating epithelial cell polarization.
CD46 plays a key role in T cell regulation. Each cytoplasmic domain differentially mediates proliferation and effector functions. Thus, CYT-1 mediates cell activation and cytokine production, while CYT-2 directs contraction of both processes. CD46 cytoplasmic domain switching connects Th1 cell activation and contraction to metabolic reprogramming pathways.

(Data source: West EE, Kolev M, Kemper C. Annu Rev Immunol. 2018)
CD46 is also a "pathogen magnet," a target of 12 pathogens, including five viruses and seven pathogens. These pathogens target different CD46 domains for attachment and entry. For example, adenovirus Ad35 binds to CCPs1-2, and it competes with measles virus MV for binding. Furthermore, after binding, CD46 can be internalized or shed. The attraction of pathogens to CD46 may include not only its widespread expression but also its immunomodulatory signaling capacity . MV (vaccine strain) downregulates IL-12 expression in monocytes by binding to CD46 .
CD46 and cancer
Complement activation is a double-edged sword in cancer: it can aid in tumor cell killing but can also promote tumor growth, inflammation, and immunosuppression. Increased expression of CD46 on malignant cells serves as an immune evasion mechanism to prevent complement activation, thereby favoring tumor cells . When cancer leads to abnormally high expression of CD46, complement's traditional role as an anti-tumor effector may be disrupted. Consequently, cytolytic complement damage (CDC) and antibody-dependent cell-mediated cytotoxicity mechanisms may be attenuated or abolished, compromising anti-tumor defenses . Upregulation of CD46 has been observed in multiple myeloma (MM) and a range of solid tumors, including those of the ovary, breast, cervix, colorectum, prostate, and bladder, and this abnormal expression may also be associated with malignant transformation and metastatic potential.
Using xenograft models, the CD46 CYT-1 isoform attenuated cell growth, migration, and tumorigenicity, whereas the CYT-2 isoform promoted these processes, suggesting that signaling pathways influenced by CD46 expression and their disruption could be a therapeutic avenue for cancer treatment.
Studies on CD46 have shown that its autocrine activation through intracellularly generated C3b, the "C3b/CD46 axis," plays a key role in nutrient uptake and enhances cellular metabolism of CD4+ T cells.

Targeting of CD46 by therapeutic viral vectors
Because CD46 is highly expressed on a variety of malignant cells and is a receptor for several adenovirus strains and the vaccine-type measles virus, it can be engineered for therapeutic applications. Therefore, modified viral vectors targeting CD46 are currently being developed for a wide range of therapeutic applications, such as Ad26 vaccine vectors for the treatment of HIV and COVID-19, as well as various forms of cancer.
CD46- targeted oncolytic adenoviral therapy
Oncolytic adenoviruses targeting CD46 (oncolytic AdVs) are an innovative cancer treatment that utilizes the oncolytic and other capabilities of CD46 combined with adenoviruses . Adenoviruses binding to CD46 may leverage its high expression on tumor cells, thereby enhancing the specificity and efficacy of treatment .
Enadenotucirev (EnAd) is a novel adenovirus group B hybrid composed of components from Ad3 and Ad11p. Its receptor binding capacity is stronger than either of its parental viruses . EnAd mediates non-apoptotic cell death by disrupting cell membranes and releasing proinflammatory mediators. It has been tested in multiple clinical trials as a monotherapy for various cancers and in combination with chemotherapy, radiotherapy, chemotherapy, and/or immunotherapy. Intravenous administration of EnAd has a manageable safety profile, stability in human blood, and the ability to increase tumor immune cell infiltration while specifically targeting cancer cells.
Newer generations of EnAds have introduced immunomodulatory or other components as transgenes ; for example, the variant NG-350A includes an agonistic anti-CD40 mAb for potential immunomodulatory and novel biological activities.
Another variant of EnAd, NG-641, encodes four immunostimulatory transgenes: a bispecific T cell activator antibody against human fibroblast activation protein ( FAP ), IFN-α2, and CXCL9 and -10 .
Oncolytic adenoviral therapies targeting CD46 can also be composed of two different adenoviruses. For example, the Ad5/35 chimeric therapy LOAd703 has been developed and is in clinical trials. It also incorporates Ad5 components spliced onto the Ad35 fiber and knob elements to target CD46. LOAd703 contains two transgenes: trimerized, membrane-bound CD40L and 4-1BB, which are directed by a CMV promoter. These modifications are designed to confer both immunostimulatory and anti-tumor activities. Clinical studies of LOAd703 in combination with other therapies are ongoing. Results from a Phase I study (NCT02705196) concluded that the data demonstrated sufficient anti-tumor activity to warrant continued trial.
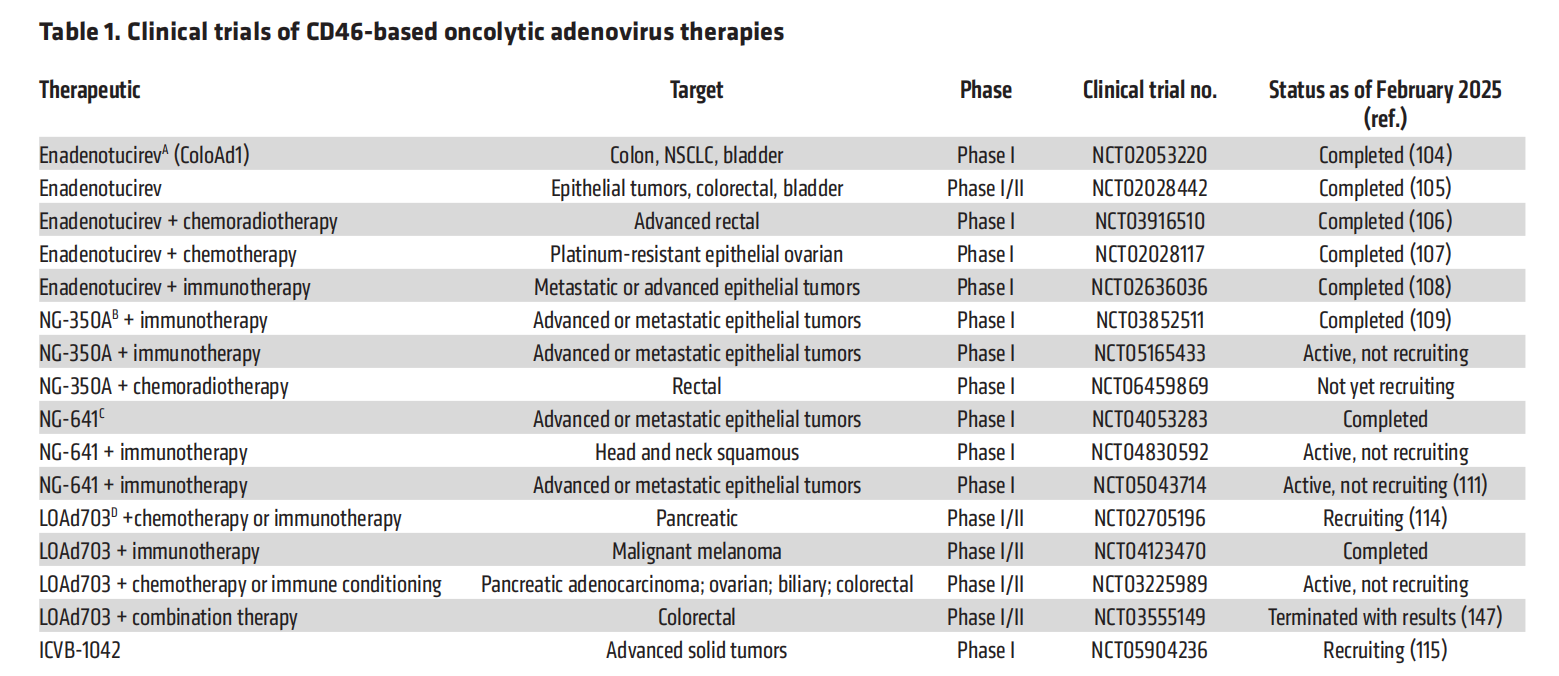
CD46-targeted MV oncolytic therapy
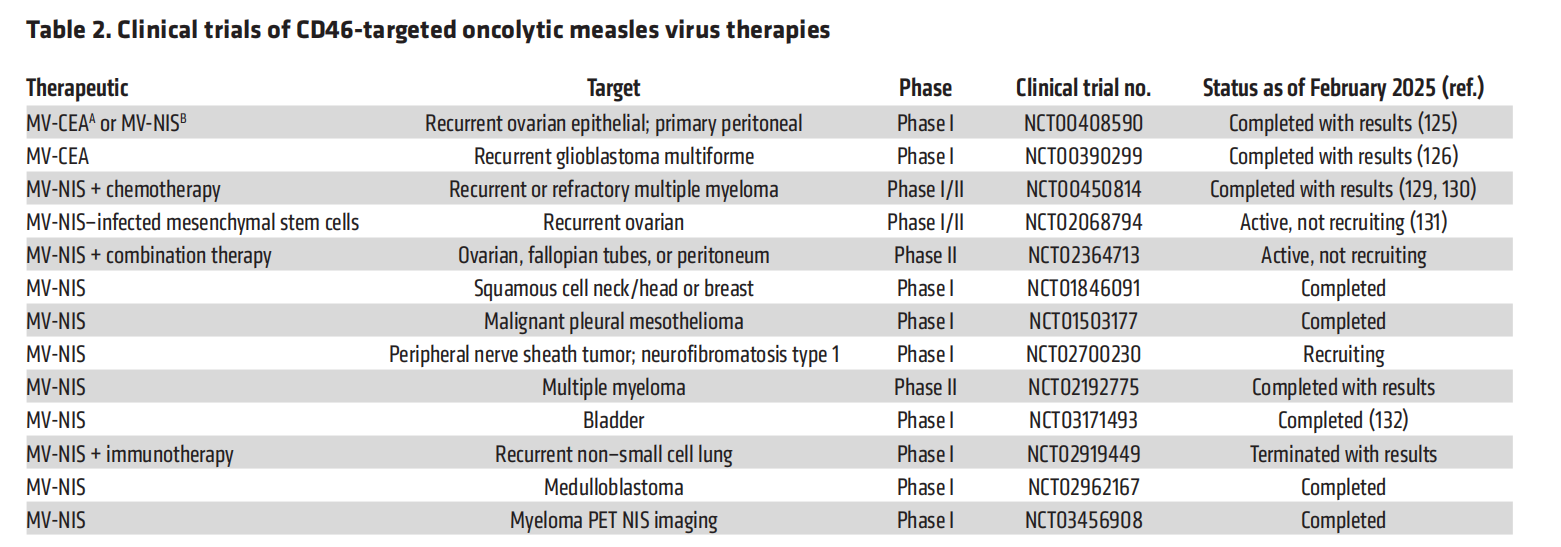
CD46 is the receptor for the laboratory-cultured MV-Edm (vaccine) strain. The advantages of using MV-Edm as an oncolytic cancer therapy include its established safety profile, its ability to replicate within and kill cancer cells, its ability to activate anti-tumor responses, and its suitability for genetic engineering. MV-Edm is able to distinguish between high CD46 density on typical tumor cells and low CD46 density on normal cells, promoting preferential killing of tumor cells. MV-Edm can exploit cell-to-cell fusion and cytoreductive cancer therapy.
Many modified MV oncolytic viral vectors have been developed and studied . MV-CEA , an engineered MV-Edm strain expressing a soluble extracellular carcinoembryonic antigen (CEA) domain, has been tested in ovarian cancer (NCT00408590) and glioblastoma (NCT00390299). These trials documented the development of tumor-specific immune responses, resulting in antitumor effects, and the treatment was well tolerated. The glioblastoma trial also demonstrated that repeated intratumoral administration was safe, with no dose-limiting toxicities.
MV-NIS is a recombinant MV-Edm vector with the addition of the human thyroid sodium-iodide symporter (NIS) gene . A study using MV-NIS for the treatment of ovarian cancer (Phase I/II, NCT02068794) also used this approach. This study found that treatment triggered cellular immunity against the tumor, was well tolerated, and was associated with a promising median overall survival (NCT03171493).
Antibody Drug Conjugates
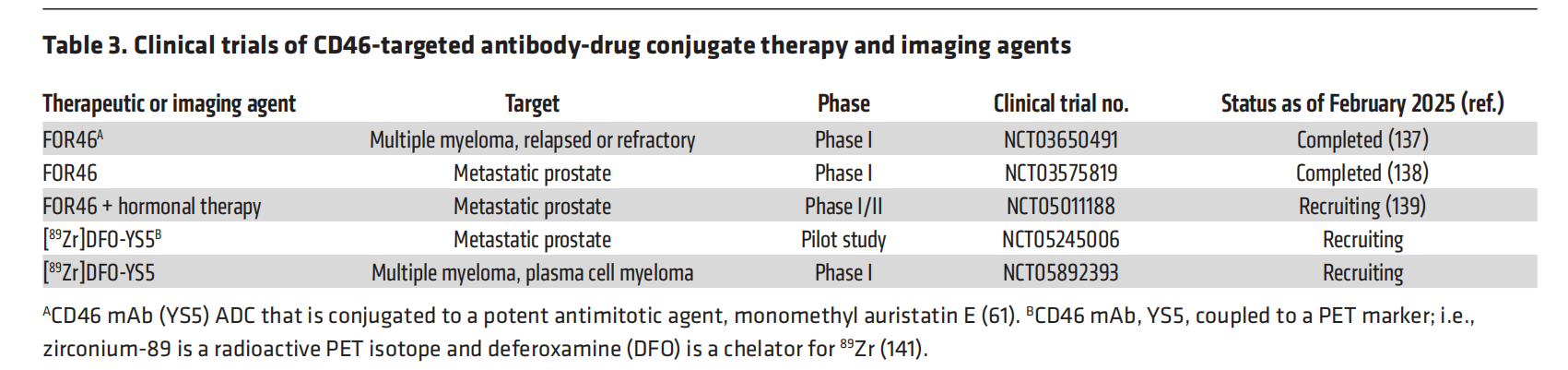
Another cancer treatment approach uses an antibody-drug conjugate (ADC) targeting human monoclonal CD46 . For example, FOR46 is being tested in multiple clinical trials for the treatment of multiple myeloma (MM) and prostate cancer , and several clinical trials using FOR46 for prostate cancer are currently underway or have been completed. A multicenter monotherapy trial (NCT03575819) determined that FOR46 was well tolerated, showed no toxicity related to CD46 targeting, and demonstrated efficacy in heavily pretreated patients.
Summarize
The future of CD46-targeted anti-cancer therapy, whether through oncolytic viruses, ADCs, or other approaches, is full of great potential. However, it faces some challenges. For example, the problem of heterogeneous tumor expression of CD46 needs to be addressed to improve treatment efficacy by reducing the possibility of triggering an immune response (existing antibodies) and improving patient stratification; ensuring tumor specificity is extremely important to reduce the bystander effect of this widely expressed protein; in-depth studies are needed to evaluate potential toxicities and verify the safety of CD46-targeted therapies in these and other aspects ; CD46-targeted approaches combined with other treatments (such as immune checkpoint inhibitors, chemotherapy, or radiotherapy) , combined with emerging innovations in gene modification and synthetic biology, may ultimately provide more effective, personalized, and safer treatment options for cancer patients.
