Growth/differentiation factor 8 (GDF8), also known as myostatin (MSTN), is a specific negative regulator of skeletal muscle (SM) growth. As a member of the TGF-β family, it may be involved in the development and occurrence of obesity, hyperlipidemia, diabetes and hypertension (clinical manifestations of metabolic syndrome), and may therefore become a potential therapeutic target for metabolic syndrome.
Expression distribution of MSTN
MSTN is mainly expressed in early spermatids, Müller glial cells, late spermatids, Schwann cells, and skeletal muscle cells.

(Data source: Uniprot)
The structure of MSTN and its receptor
MSTN is a secreted protein consisting of 375 amino acids. The MSTN precursor protein is cleaved by the furin protease, generating an N-terminal propeptide and a C-terminal dimer, the actual signaling molecule. The propeptide remains non-covalently bound to the C-terminal dimer, maintaining MSTN in an inactive, latent state. Latent MSTN is activated by cleavage of the propeptide by metalloproteases from the BMP-TLD family at the N-terminal end of aspartate 76, enabling the C-terminal dimer to bind to cell surface receptors.

(Data source: Lee SJ. J Clin Invest. 2021)
Pro-MSTN is a homodimer composed of two identical disulfide-linked subunits. Each chain consists of 109 amino acid residues and contains a prodomain ( N- terminus) and a smaller growth factor (GF) domain ( C -terminus). Like other TGF-β superfamily members, the GF domain of MSTN contains a cystine knot motif and four antiparallel β strands, termed "fingers." The two identical GF domains of MSTN are linked by their concave "palms," covalently linked by a disulfide bond between residues C339 in the wrist region. The prodomain contains an N -terminal "forearm" helix that grasps the mature GF and a globular "arm/shoulder" domain that sits atop the mature GF protomer. Each MSTN monomer has four intermolecular disulfide bonds, three of which participate in the formation of the cysteine knot. When two MSTN monomers assemble in an antiparallel orientation, they create either a convex or concave surface. Cysteine and dimerization are the primary determinants of MSTN stability.

(Data source: Cotton TR, et al. EMBO J. 2018)
Signaling pathway and regulation of MSTN
The mechanism of MSTN-induced muscle loss is mediated by decreased protein synthesis and/or increased protein catabolism. MSTN reduces protein synthesis by inhibiting the Akt/mTOR signaling pathway and induces muscle atrophy by promoting the transcription of atrophy-related genes (atrogenes). The MSTN signaling pathway can be divided into Smad-mediated and non-Smad-mediated pathways.
Smad -mediated signaling pathway
The C-terminal dimer of mature MSTN binds to ACVRIIB, leading to the recruitment and activation of activin type I receptors (Alk4 or Alk5), which in turn promotes the activation of Smad2 and Smad3 to inhibit myoblast differentiation. Activation of the MSTN signaling pathway inhibits Akt phosphorylation through IGF-1 and may mediate IGF-1-induced protein synthesis through Smad3.
MSTN-mediated non-Smad signaling pathway
The non-Smad pathway of MSTN acts synergistically through multiple pathways such as MAPK, Wnt, AKT/mTOR and FOXO to inhibit myoblast proliferation and differentiation; reduce protein synthesis; and activate the protein degradation system.

(Data source: Baig MH, et al. Front Physiol. 2022)
Targeted therapy for MSTN
Targeted therapy for MSTN mainly increases muscle mass and improves metabolism by inhibiting its signaling pathways. Currently, many types of biological agents have been developed and clinical trials are being conducted in various diseases.
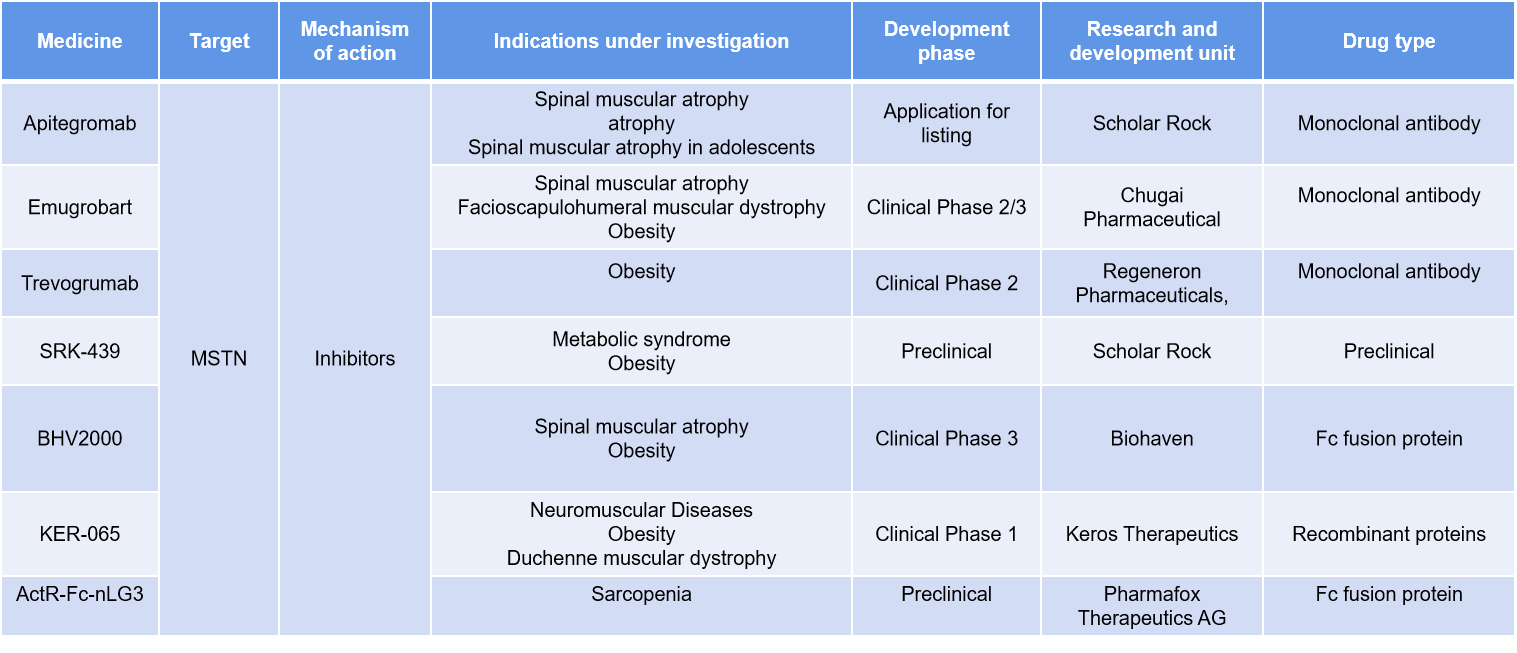
(Data source: Synapse)
Apitegromab , developed by Scholar Rock, is a monoclonal antibody targeting MSTN and is in the marketing application stage for the treatment of spinal muscular atrophy (SMA). Unlike most myostatin inhibitors, apitegromab selectively targets the latent or inactive form of myostatin, inhibiting MSTN before its release to block its activation in the muscle. Its purpose is to maintain or increase motor function in patients currently receiving existing SMA therapies, which can slow further motor neuron degeneration but cannot directly address muscle atrophy. Scholar Rock reported positive top-line data from the SAPPHIRE clinical trial in October 2024, showing that 30% of patients treated with Apitegromab had a 3-point improvement in HFMSE, compared to only 12.5% in the placebo group.

(Data source: Scholar Rock)
Emugrobart (GYM329 /RG6237) is a Chugai-developed monoclonal antibody targeting MSTN. Using a retrieval and scanning antibody technology, it eliminates latent myostatin from plasma and tissues. Latent myostatin is an inactive form primarily secreted by muscle cells and activated by BMP-1 and other protein-degrading enzymes. Activated myostatin inhibits muscle growth and hypertrophy. By inhibiting latent myostatin, GYM329 offers a more targeted approach and is expected to improve various conditions associated with muscle atrophy and loss of strength.
Taldefgrobep alfa (BHV2000) is a MSTN-targeted Fc-fusion protein developed by Biohaven that can directly reduce myostatin and block key downstream signaling mechanisms. Taldefgrobep is an investigational muscle-targeted recombinant protein that, when used in combination with other approved therapies, has the potential to enhance muscle mass and strength in patients with SMA. Data from previous clinical studies in healthy volunteers and patients with Duchenne muscular dystrophy have demonstrated that taldefgrobep alfa is generally safe and well-tolerated.

(Data source: Biohaven official website)
