Background
Glycosylation is a key protein modification in vivo, playing an important role in regulating various biological functions by influencing protein folding, transport, and localization. Changes in glycosylation patterns are a key hallmark of cancer, associated with a range of pathological events in cancer-related processes. They serve as important biomarkers and provide new targets for cancer diagnosis and treatment. Glycoproteins such as human epidermal growth factor receptor 2 (HER2) for breast cancer, alpha-fetoprotein (AFP) for liver cancer, carcinoembryonic antigen (CEA) for colon cancer, and prostate-specific antigen (PSA) for prostate cancer are all approved tumor biomarkers for clinical use.
On September 21, 2024, researchers from the Department of Oncology at People's Hospital of Hunan Province published an article titled "Altered glycosylation in cancer: molecular functions and therapeutic potential" in Cancer Communications (London, England). The article describes the diversity of glycosylation structures and the newly discovered glycosylation substrate—glycosylated RNA (glycoRNA). The article primarily explores the role of glycosylation in cancer from the perspectives of tumor metastasis, immune evasion, metabolic reprogramming, aberrant ferroptosis, and cellular senescence. Furthermore, the article summarizes the clinical applications of protein glycosylation in cancer diagnosis, treatment, and multidrug resistance, and provides an outlook on the clinical application prospects of protein glycosylation.
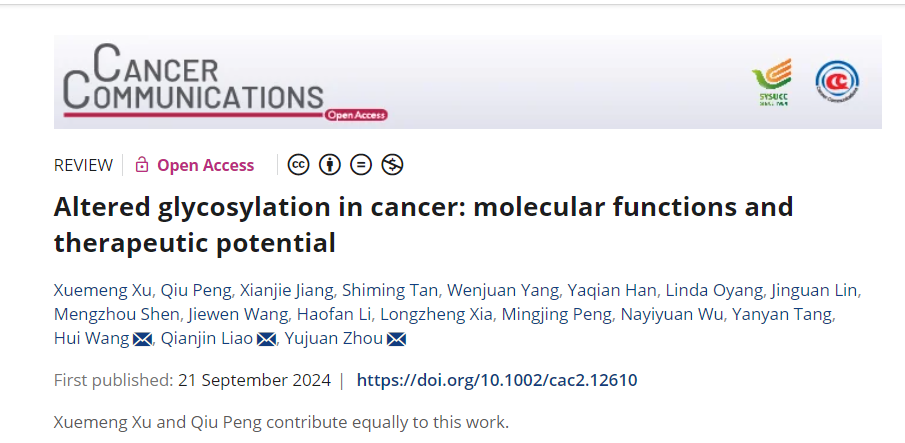
The structure and biological function of glycosylation
Protein glycosylation is the attachment of sugar chains to certain amino acids on a protein by glycosyltransferases and diphosphoinositides. Glycosylation facilitates proper protein folding and plays important roles in biology, including receptor interactions and facilitating intercellular signaling. Glycosylation can be categorized by site, including N-glycosylation, O-glycosylation, C-glycosylation, and glycosylphosphatidylinositol.
N-Glycosylation
O-Glycosylation refers to the covalent attachment of N-acetylglucosamine (GlcNAc) in N-glycans to the nitrogen atom of the asparagine (Asn) amino group via a β1-glycosidic bond. This change typically occurs at specific Asn sites within the conserved glycosylation pattern Asn-X-Ser/Thr, where " X " represents any amino acid other than proline. N-linked glycosylation is a tightly regulated process involving a series of coordinated post-translational events that lead to the modification and alteration of cell surface, secreted, and circulating proteins. N-linked glycan synthesis is closely linked to numerous roles in protein folding, endoplasmic reticulum homeostasis, and lysosomes and autophagy. Proteins lacking N-glycosylation are not properly recognized by calnexin (CNX) and calreticulin (CRT) in the endoplasmic reticulum, leading to misfolding and degradation of hypoglycosylated proteins.
O-glycosylation
O-glycosylation occurs when a polysaccharide attaches to the hydroxyl group of a serine or threonine residue in a polypeptide. The primary sugars attached to serine and threonine amino acids are N-acetylglucosamine (GlcNAc) and N-acetylgalactosamine (GalNAc). O-GlcNAc glycosylation influences the conformation and stability of protein structure, mediating protein-protein interactions and molecular recognition by biological receptors.
C-Glycosylation
C- glycosylation is a relatively rare glycosylation modification consisting of an α-D-mannopyranosyl group attached to a tryptophan ( Trp ) residue via a C-C glycosidic bond. The amino acid sequence of the C-glycosylation site is Trp-XX-Trp/Cys/Pro, where X represents any amino acid. C-glycoproteins account for approximately 20% of proteins that are secreted or embedded in cell membranes. C-mannosylation is crucial for proper protein folding, secretion, and stability.
Glycosylphosphatidylinositol
Glycosylphosphatidylinositol ( GPI ) is a complex glycolipid . The GPIT complex is a transmembrane enzyme that facilitates the binding of GPI to proteins within the endoplasmic reticulum, thereby aiding the maturation of GPI-anchored proteins (GPI-APs). GPI-AP proteins are involved in other biological processes, such as biosynthesis, trafficking, cell membrane distribution and signal transduction, cell adhesion, and antigen presentation.
GlycoRNA
Glycosylation refers to the glycosylation that occurs on cell surface RNA. Glycosylated RNA can serve as a direct ligand for cell membrane Siglec receptors. Siglec receptors play an important role in immune regulation, and glycosylated RNA plays a crucial role in various physiological and pathological processes, such as host immune responses, tumor evasion of the immune system, and autoimmune diseases. GlycoRNA can promote intercellular communication in the immune system. Cell surface glycoRNA is crucial for neutrophil recruitment. P-selectin (SELP) promotes communication between neutrophils and endothelial cells, enabling their recognition.
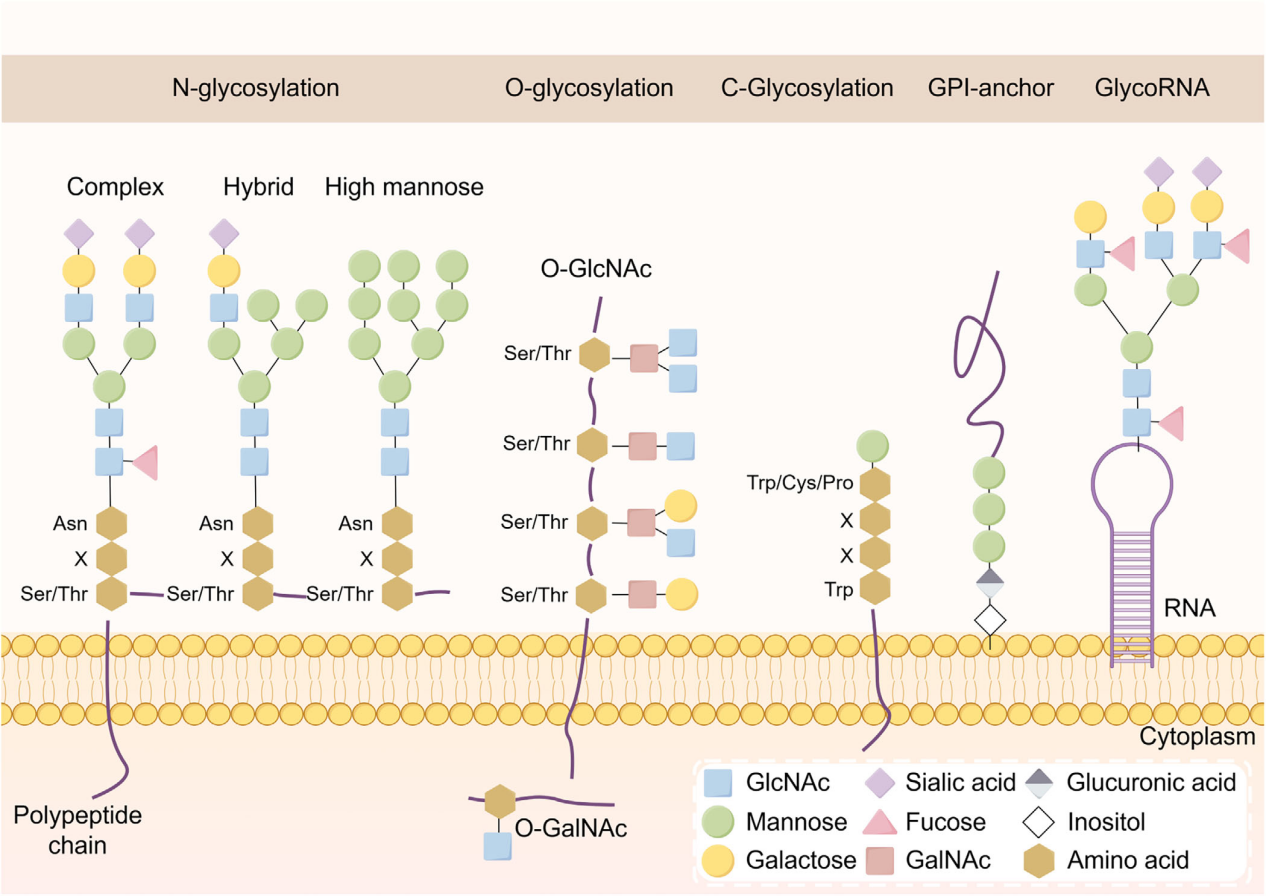
The role of glycosylation in cancer cells
Glycosylated proteins are involved in a variety of biological processes within cells. Abnormal glycosylation is closely related to many disease processes. When glycosylation is not properly regulated in cancer, it can lead to disrupted signal transduction, cancer cell spread, and evasion of immune system detection.
Glycosylation and tumor metastasis
Disruption of glycosylation, particularly in glycan structure, glycosyltransferase activity, and expression of glycosylated substrates, can affect events associated with tumor progression, such as cancer stem cell (CSC) characteristics and epithelial-mesenchymal transition (EMT). It can also impact a range of oncogenic signaling pathways, triggering tumor invasion and metastasis. For example, B3GNT5 glycosylation is associated with cancer stem cell properties in basal-like breast cancer. SLC35A2 promotes HCC metastasis by regulating glycosylation to increase cell adhesion.
Glycosylation and tumor immune escape
As cancer progresses, tumor cells adapt to the selective pressures of the immune system, gradually developing methods to evade detection. Tumor cell glycosylation plays a key role in circumventing effective immune responses. For example, GALNT7 modifies O-glycosylation associated with immune signaling pathways in prostate cancer cells . In breast cancer cells, O-linked sialoglycosylation of CD55 by ST3GAL1 contributes to immune evasion. FUT8-mediated aberrant B7H3 glycosylation suppresses immune responses in TNBC cells. B4GALT1 mediates N-linked glycosylation of the PD-L1 protein, thereby preventing PD-L1 degradation at the posttranscriptional level. TGF-β1-mediated PD-L1 glycosylation promotes immune evasion through the c-Jun/STT3A signaling pathway. TMUB1 enhances PD-L1 N-glycosylation and stability by recruiting STT3A, thereby promoting PD-L1 maturation and tumor immune evasion. O-GlcNAcylation impedes lysosomal degradation of PD-L1, promoting tumor immune evasion.

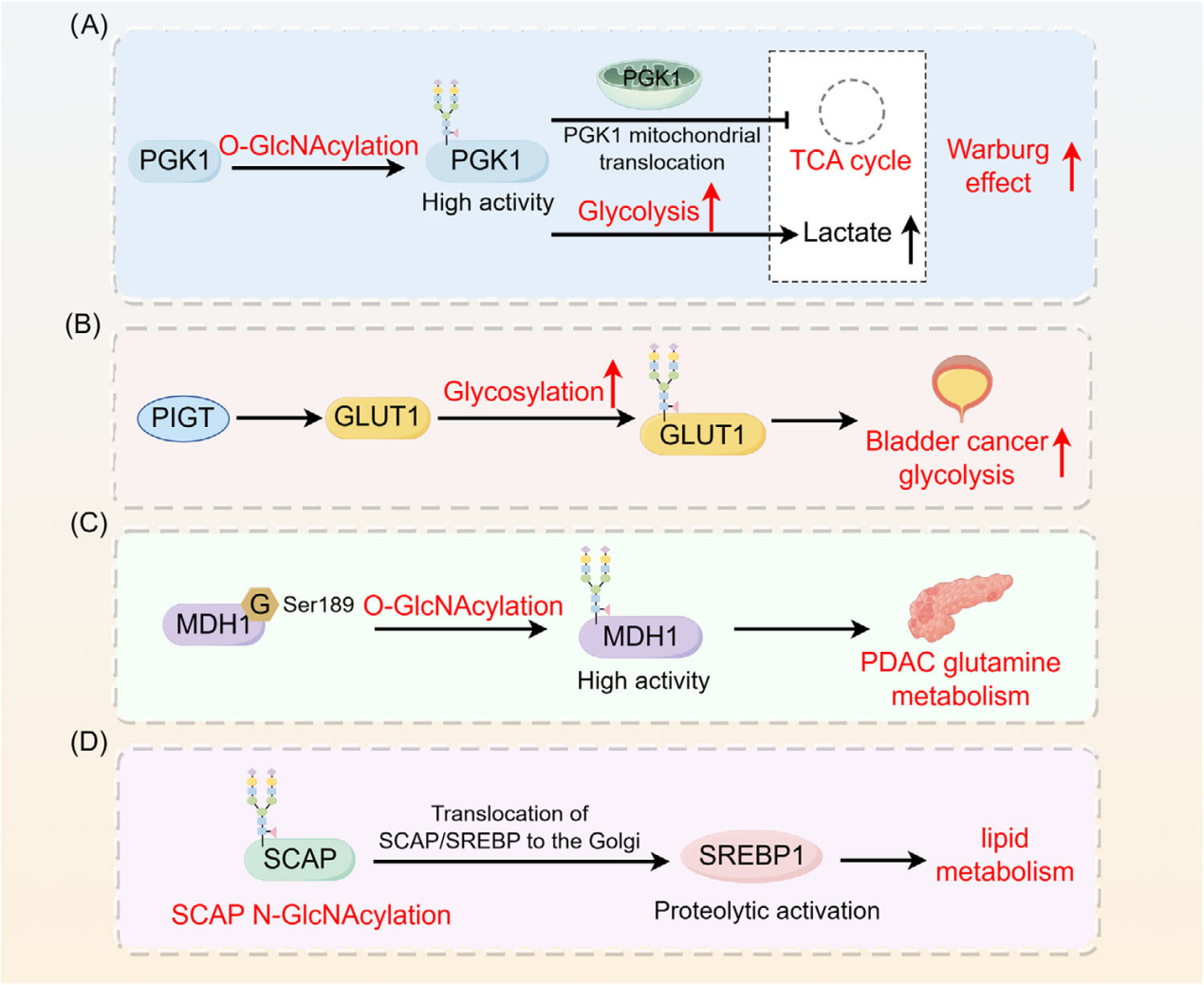
Glycosylation and tumor metabolic reprogramming
Protein glycosylation is crucial for metabolic reprogramming in cancer cells, orchestrating metabolic pathways to produce essential metabolites that promote tumor progression . Glycosylation increases the metabolic enzyme activity of PGK1 and induces its translocation to mitochondria to inhibit the TCA cycle, thereby enhancing the Warburg effect in cancer cells. PIGT enhances glycolysis in bladder cancer cells by regulating GLUT1 glycosylation. O-GlcNAcylation regulates the metabolic activity of MDH1, promoting glutamine metabolism in pancreatic cancer. SCAPN-glycosylation promotes the translocation of SCAP/SREBP to the Golgi apparatus, thereby activating SREBP1 and regulating lipid metabolism in tumors through SREBP-dependent lipid transport.
Glycosylation and ferroptosis
Glycosylation interacts with ferroptosis in cancer and regulates tumor progression. Deglycosylation of the ferritin heavy chain enhances its interaction with the ferritin phagocytic receptor NCOA4, leading to the accumulation of labile iron in mitochondria and ferroptosis. Inhibition of N-glycosylation of 4F2hc enhances the ferroptosis sensitivity of PDAC cells by inhibiting the activity of the glutamate-cystine antiporter Xc-. USP8 suppresses ferroptosis sensitivity in hepatocellular carcinoma by stabilizing OGT, which promotes O-GlcNAcylation of SLC7A11. Glucose-induced O-GlcNAcylation of ZEB1 activates the transcriptional activity of the lipogenesis-related genes FASN and FADS2, leading to lipid peroxidation-dependent ferroptosis in mesenchymal pancreatic cancer cells.

Glycosylation and cell aging
Senescent cells evade immune surveillance and may promote tumorigenesis and progression through mechanisms such as DNA damage and oxidative stress. The cell-permeable inhibitor NGI-1 targets oligosaccharyl transferase, blocking cell surface localization and EGFR glycoprotein signaling, and promoting cell senescence. Increased O-GlcNAcylation levels in KRAS-mutant lung cancer cells inhibit KrasG12D OIS and accelerated lung tumorigenesis.

The therapeutic potential of glycosylation in cancer
Glycosylation-based tumor biomarkers
Many glycoproteins, such as AFP for liver cancer, CEA for colon cancer, and prostate-specific antigen (PSA) for prostate cancer, have been clinically approved as tumor biomarkers. Hyperglycosylated hCG is a marker of early invasiveness in human trophoblasts. Aberrant expression of glycosyltransferases and glycosidases plays a key role in the disruption of glycan chains and glycoproteins. Therefore, these enzymes can be considered as potential biomarkers for cancer. For example, POFUT1 in gastric cancer and GCNT2 in melanoma have both been proposed as tumor biomarkers.

Glycosylation and tumor therapy
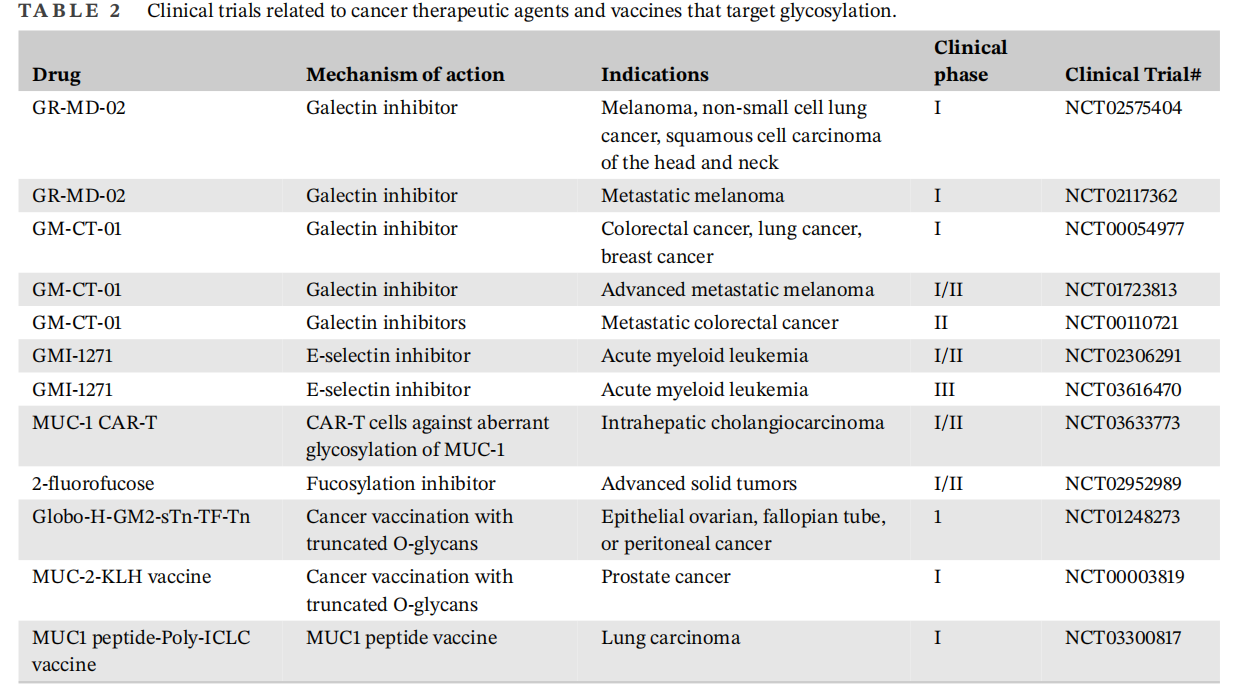
Studies have found that glycosylation is crucial in the emergence of acquired resistance to multiple drugs. Tunicamycin is an effective glycosylation inhibitor that has shown significant anti-tumor effects in various types of tumors through glycosylation inhibition. Targeted inhibition of tumor glycosylation is a feasible method to overcome chemotherapy resistance in gastric cancer patients. Targeting the glycosylation of MUC1 is a promising strategy to improve gastric cancer resistance. Interfering with the N-glycosylation of tumor cells can significantly improve the efficacy of CAR-T cell therapy for solid tumors. ALG3 has been shown to increase radioresistance in breast cancer patients and promote the growth of tumor stem cells by modifying the glycosylation of TGFBR2. Currently, many drugs and vaccines targeting glycosylation are undergoing clinical trials for the treatment of cancer.
Summarize
Glycosylation influences protein folding and plays a crucial role in biological function and tumor immunotherapy. The study of aberrant glycosylation offers new insights into cancer progression . Studying the mechanisms of aberrant glycosylation and identifying glycoprotein biomarkers will contribute to advances in cancer treatment, providing scientific guidance for early detection and treatment. Future advances in glycomics and glycosylation research may significantly improve the current cancer treatment landscape.
