c-MET, also known as MET or hepatocyte growth factor receptor (HGFR), is a receptor tyrosine kinase belonging to the MET family. Hepatocyte growth factor (HGF) is a specific ligand for c-MET, and binding to it activates intracellular signaling pathways that regulate numerous physiological processes, including proliferation, scattering, morphogenesis, and survival. Aberrant activation of c-MET can lead to tumor growth and metastatic progression of cancer cells, making it an important drug target for cancer treatment.
c-MET expression distribution
c-MET is mainly expressed in glandular and luminal cells, salivary duct cells, type 2 alveolar cells, cytotrophoblast cells, and mesothelial cells.
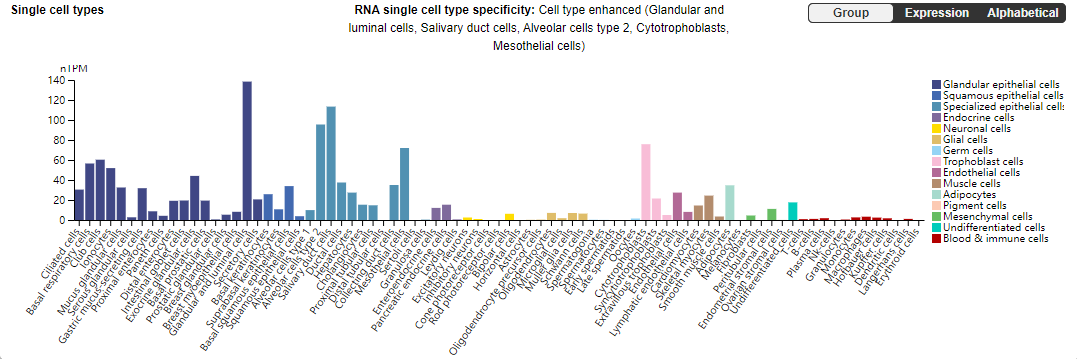
(Data source: uniprot)
Structure of c-MET
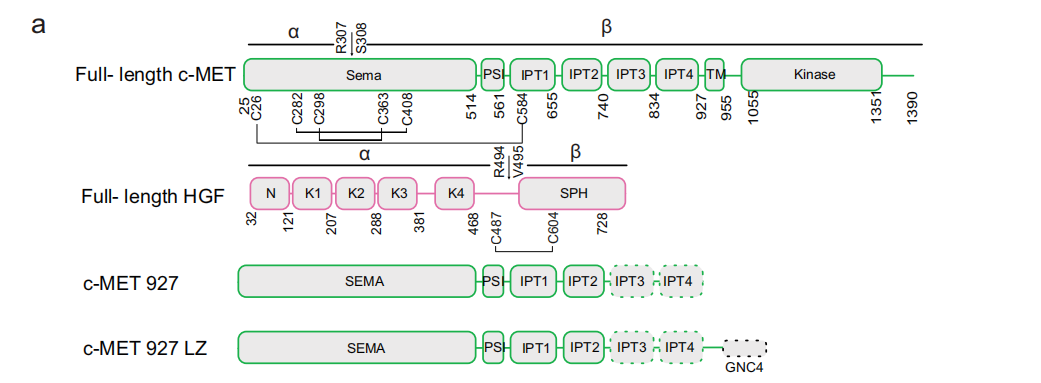
c-MET is a type I membrane protein. The c-MET receptor consists of a semaphorin (SEM A) domain, a plexiform semaphorin integrin (PSI) domain, four contiguous immunoglobulin-plexiform semaphorin transcription factor (IPT1-4) domains in the extracellular region, a transmembrane helix (TM), and an intracellular KD. c-MET is initially expressed as a 150 kDa single-chain precursor that is converted to its mature form by furin proteolysis between Arg307 and Ser308. The mature form contains α- (32 kDa) and β- (120 kDa) subunits linked by at least three disulfide bonds. Similar to c-MET, proteolytic cleavage of HGF between Arg494 and Val495 generates α and β subunits (57 and 26 kDa, respectively). Both pro-HGF and cleaved HGF bind to c-MET with high affinity, but only cleaved HGF activates the c-MET signaling pathway.
c-MET forms an asymmetric 2:2 complex with HGF. One HGF molecule can link two c-MET molecules together, forming a minimal 2:1 c-MET: HGF active complex. This minimal active complex is further stabilized by a second HGF molecule and heparin, forming an even more stable 2:2 complex, thereby enhancing c-MET activation.

(Data source: Uchikawa E, et al. Nat Commun. 2021)
c-MET signaling pathway and regulation:
In the canonical pathway, HGF binding induces the dimerization of two c-Met molecules, leading to autophosphorylation of tyrosine residues and activation of numerous downstream signaling pathways, such as the MAPK/ERK, STAT3, and PI3K/AKT pathways. JNK is also phosphorylated and activates multiple downstream substrates, including transcription factors such as AP-1 and apoptosis-related Bcl-2 and Bax. These factors drive a wide range of cellular phenotypes, including morphogenesis, survival, proliferation, motility, invasion, and metastasis. Non-canonical pathways are activated when c-Met binds to other receptors (EGFR, MUC-1, VEGFR, CD44, Plexin B1, HER, and Integrin).

(Data source: Liu X, et al. Front Cell Dev Biol. 2020)
c-MET targeted therapy
Therapeutic intervention strategies to block and inhibit the MET receptor oncogenic signaling cascade include blocking ligand-receptor interactions, preventing receptor dimerization, blocking MET kinase intrinsic activity, and inhibiting specific downstream signaling pathways. Currently, many monoclonal antibodies, bispecific antibodies, and ADCs targeting c-MET are in clinical trials for the treatment of non-small cell lung cancer, colorectal cancer, gastrointestinal tumors, and other diseases.
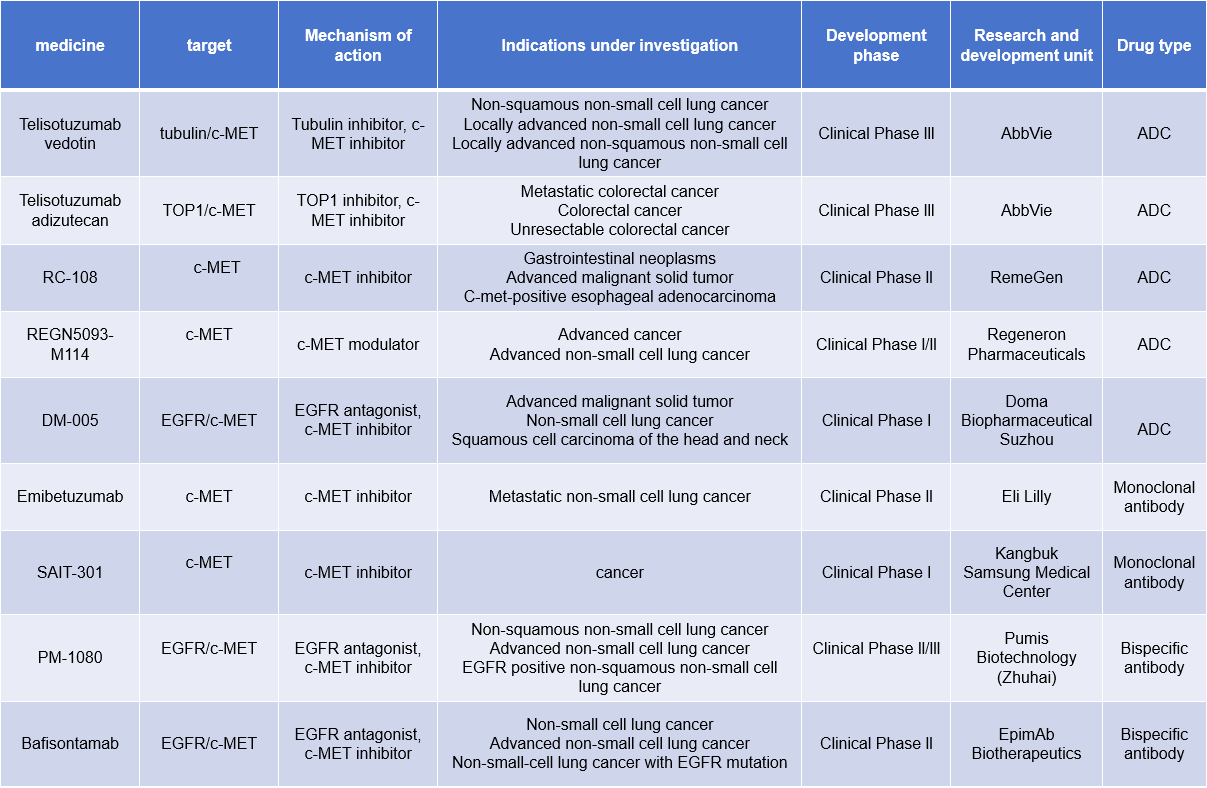
(Data source: New Drug Intelligence Database)
Telisotuzumab vedotin (ABBV-399) is an antibody-drug conjugate targeting c-MET developed by AbbVie and is currently in Phase 3 clinical trials for the treatment of non-small cell lung cancer.
Telisotuzumab adizutecan (ABBV-400), another ADC being investigated by AbbVie, carries a TOP1 inhibitor as its payload and is in Phase 3 clinical trials for the treatment of metastatic colorectal cancer, colorectal cancer, and unresectable colorectal cancer. ABBV-400 uses c-Met to deliver a topoisomerase inhibitor to cells overexpressing it, thereby inhibiting DNA replication; this helps to halt cell proliferation and induce cytotoxicity.

(Data source: abbvie official website)
Emibetuzumab is a humanized bivalent anti-c-Met IgG4 monoclonal antibody being developed by Eli Lilly and Company for the treatment of metastatic non-small cell lung cancer in Phase 2 clinical trials. It works by blocking the binding of hepatocyte growth factor (HGF) to c-Met. This blockade inhibits activation of both HGF-dependent and -independent MET signaling pathways, thereby suppressing tumor growth.
Bafisontamab is a bispecific antibody targeting EGFR/c-MET developed by EpimAb Biotherapeutics for the treatment of non-small cell lung cancer, advanced non-small cell lung cancer, and EGFR-mutated non-small cell lung cancer. It is in Phase 2 clinical research.
