B-cell activating factor receptor (BAFFR), also known as TNFRSF13C and CD268, is a member of the tumor necrosis factor receptor (TNFR) superfamily. It is primarily expressed on the surface of mature and activated B cells and plasma cells and is a core receptor regulating B cell development, survival, and differentiation. 13 Its ligand, BAFF (B-cell activating factor), binds to BAFFR, transmitting survival signals and maintaining B cell homeostasis. Dysfunction of BAFFR can lead to immunodeficiency, autoimmune diseases, or B-cell malignancies.
BAFFR expression distribution
BAFFR is highly expressed in the spleen and lymph nodes, and in resting B cells. Lower levels of expression are detected in activated B cells, resting CD4+ T cells, the thymus, and peripheral blood leukocytes.

(Data source: uniprot)
Function of BAFFR
Maintaining B cell survival: by activating the NF-κB non-canonical pathway (dependent on the TRAF2/3-NIK signaling axis), upregulating anti-apoptotic proteins (such as BCL-2 and MCL-1), and promoting the long-term survival of mature B cells;
Mediating B cell differentiation: Coordinated BCR signaling drives B cells to differentiate into plasma cells, supporting antibody production;
Regulating immune tolerance: its absence leads to apoptosis of transitional B cells and breaks autoimmune tolerance (e.g., pathogenic B cell expansion in SLE);
Involvement in pathological processes: Excessive activation can trigger autoimmune diseases (rheumatoid arthritis, lupus) and B- cell lymphomas (such as DLBCL ). Clinically targeted drugs (such as the anti- BAFFR monoclonal antibody Ianalumab ) treat related diseases by blocking this pathway .
The structure of BAFFR
BAFFR is a 184 -amino acid protein encoded by the TNFRSF13C gene and a member of the tumor necrosis factor receptor superfamily (TNFRSF) . This receptor is a transmembrane protein composed of several functional extracellular and intracellular domains. The extracellular domain contains a unique shortened CRD domain (only four conserved cysteines), which forms a compact binding interface through disulfide bonds and specifically binds to BAFF trimers but not APRIL. The transmembrane domain relies on ligand-induced dimerization to transmit signals. The intracellular domain is extremely short (68 aa) and contains the key PVIET motif that recruits TRAF2/3 (without the death domain), specifically activating the non-canonical NF-κB pathway.

(Data source: AlphaFold)
BAFFR signaling pathway and regulation
NF-κB pathway (non-canonical pathway):
After BAFF binds to BAFFR, it recruits TRAF3 and activates the kinase NIK, which in turn phosphorylates IKKα. Activated IKKα promotes the degradation of p100 within the RelB-p100 complex to p52. The RelB-p52 heterodimer then translocates to the nucleus, regulating the expression of anti-apoptotic genes such as BCL-2. Function: Promotes B cell survival and inhibits apoptosis.
PI3K/AKT/mTOR pathway:
BAFFR recruits PI3K through TRAF2/6, catalyzing the conversion of PIP2 to PIP3 and activating AKT. Downstream, phosphorylation of mTOR regulates metabolic reprogramming, enhancing mitochondrial function and inhibiting apoptotic proteins (such as BAD and FOXO)1810. Function: Supports B cell proliferation and energy metabolism, directly driving abnormal B lymphocyte expansion in rheumatoid arthritis (RA) models.
MAPK pathway:
BAFFR activates ERK1/2, JNK, and p38 mitogen-activated protein kinase (MAPK) through TRAFs. ERK1/2 promotes cell cycle progression (e.g., Cyclin D1 expression); JNK/p38 regulates inflammatory responses and cell migration. Function: Involved in B cell differentiation, release of inflammatory cytokines (e.g., IL-6, IL-1β), and tissue infiltration. Pathway interactions: These three pathways work synergistically to form a "proliferation-anti-apoptosis-inflammation" network, which is particularly aberrantly activated in autoimmune diseases (e.g., SLE, RA) and B cell lymphomas.
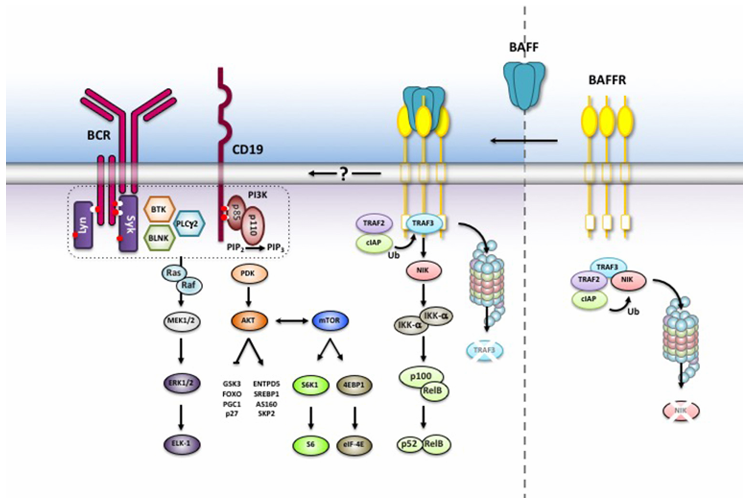
(Data source: Cristian R Smulski 1, Hermann Eibel. Front Immunol. 2018)
BAFFR and disease
Autoimmune diseases: Overactivation of BAFFR leads to the expansion of autoreactive B cells and the production of autoantibodies, ultimately leading to the development of diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and Sjögren's syndrome (SS);
Immunodeficiency diseases: Gene mutations (such as P21R) cause B cell developmental arrest, which is seen in common variable immunodeficiency (CVID) and selective IgA deficiency;
B-cell malignancies: Sustained activation of the NF-κB/PI3K pathway promotes tumor survival, as exemplified in diffuse large B-cell lymphoma (DLBCL) and chronic lymphocytic leukemia .

(Data source: Samy E, Wax S, Huard, et al. Int Rev Immunol. 2017)
BAFFR-targeted therapy
Ianalumab ( NOV-5 / VAY-736 ), a fully humanized monoclonal antibody targeting BAFFR , developed by Novartis , is a BAFFR -blocking antibody that specifically binds to BAFF-R on the surface of B cells , blocking the interaction between endogenous BAFF ( B- cell activating factor) and the receptor. It is used to treat conditions such as Sjögren's syndrome, systemic lupus erythematosus, immune thrombocytopenia, and febrile autoimmune hemolytic anemia. On February 15, 2018 , Ianalumab first began Phase III clinical trials in the United States for the treatment of autoimmune hepatitis.
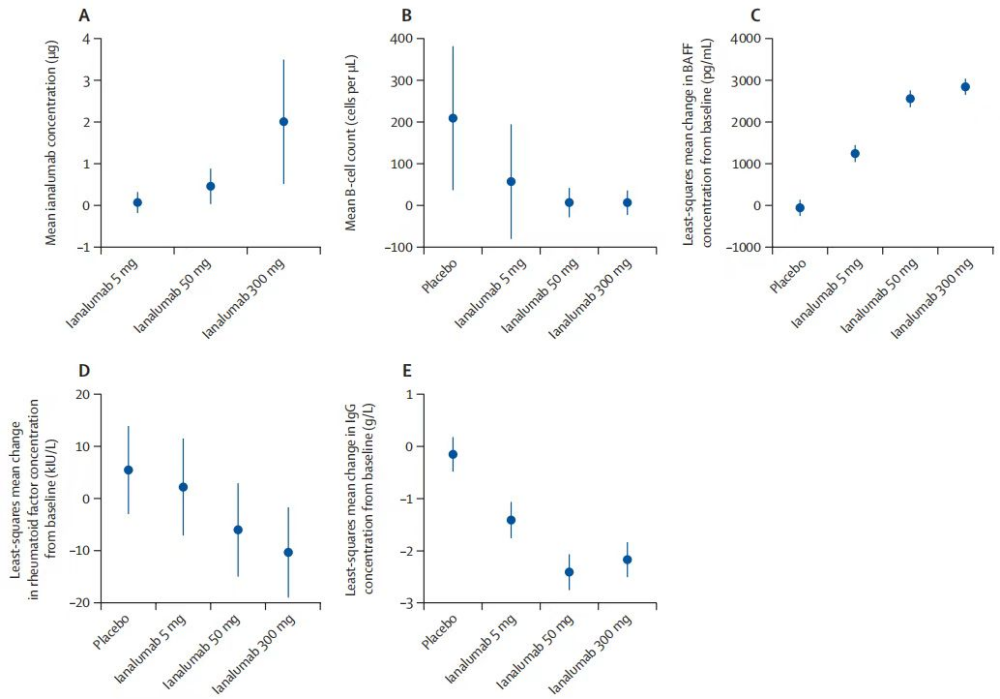
(Data source: Bowman SJ, et al. Lancet. 2022)
Telitacicept is a recombinant human B lymphocyte stimulator receptor-antibody fusion protein developed by RemeGen Co., Ltd. that targets APRIL and BAFF. BAFF and APRIL are core regulatory factors for B cell maturation and differentiation. Overexpression can lead to abnormal B cell activation and autoantibody production. Tacicept can efficiently bind to both, blocking their binding to receptors (TACI/BCMA/BAFF-R), thereby inhibiting B cell proliferation, plasma cell differentiation, and the production of pathogenic autoantibodies (such as Gd-IgA1). It is used to treat myasthenia gravis, rheumatoid arthritis, systemic lupus erythematosus and other diseases. On March 9, 2021, it was first approved for marketing in China for the treatment of diseases such as the immune system.
Anti-BAFFR CAR-T, a BAFFR-targeted CAR-T therapy (Patent No. CN119241730A ) developed by Tianjin Medical University General Hospital couples an anti- BAFFR nanobody with a second-generation signaling domain ( CD3ζ/4-1BB ). Its mechanism of action is to secrete IL-10 in BAFFR CAR-T cells , enhancing anti-tumor activity and reducing T cell exhaustion, thereby inducing T cell-specific B cell cytotoxicity. It is intended for the treatment of myasthenia gravis and aquaporin 4 antibody-positive neuromyelitis optica . The first Phase 2 clinical trial was conducted on October 1, 2024.
