Thymic stromal lymphopoietin (TSLP) is a pleiotropic cytokine that acts on multiple cell lineages, including dendritic cells, T cells, B cells, neutrophils, mast cells, eosinophils, and innate lymphocytes, influencing their maturation, survival, and recruitment. It is a key mediator of type 2 immune responses and a promoter of T helper 2 (TH2) cell-mediated diseases, including asthma and atopic dermatitis (AD). It also plays an important role in cancer and chronic inflammatory diseases.
The origin and targets of TSLP
A variety of environmental factors, including mechanical injury, Toll-like receptor (TLR) ligands, viruses, and cytokines, induce the production of thymic stromal lymphopoietin (TSLP). Epithelial cells are the primary source of TSLP. Fibroblasts, dendritic cells (DCs), basophils, and mast cells also produce TSLP upon stimulation. TSLP has pleiotropic effects on B cells, T cells, eosinophils, group 2 innate lymphoid cells (ILC2s), natural killer T (NKT) cells, macrophages, smooth muscle cells, and neurons, and also has effects on DCs, basophils, and mast cells. TSLP acts as an alarmin, rapidly released from cells to trigger further exogenous and endogenous danger signals, exacerbating inflammation.

(Data source: Ebina-Shibuya R, et al. Nat Rev Immunol. 2023)
Structure of TSLP
TSLP has two isoforms: long (lfTSLP) and short (sfTSLP). sfTSLP consists of 63 amino acids, while lfTSLP consists of 159 amino acids. TSLP has a typical short-chain four-α-helical bundle structure, which is characteristic of type I IL-2 family cytokines. TSLP can bind to its receptor, TSLPR (thymic stromal lymphopoietin receptor), to form a binary complex. This complex then binds to the IL-7Rα chain to form a ternary signaling complex, activating intracellular signaling pathways.

(Data source: Verstraete K, et al. Nat Commun. 2017)
TSLP signaling pathway and regulation:
Thymic stromal lymphopoietin (TSLP) binds to a ternary complex consisting of the TSLP receptor (TSLPR) and the IL-7 receptor α chain (IL-7Rα). TSLP activates JAK1 (via IL-7Rα) and JAK2 (via TSLPR). JAK1 and JAK2 primarily activate the signal transducers and activators of transcription STAT5A and STAT5B, and to a lesser extent STAT1 and STAT3, ultimately driving the production of IL-4, IL-5, IL-9, and IL-13, and their proinflammatory effects.

(Data source: Ebina-Shibuya R, et al. Nat Rev Immunol. 2023)
TSLP and disease
TSLP, along with other epithelial cell-derived cytokines IL-25 and IL-33, plays a key role in the development of allergic diseases, including asthma, Alzheimer's disease, and food allergies. Exposure of epithelial cells to allergens, microorganisms, and chemicals stimulates the release of TSLP. TSLP promotes and amplifies T helper 2 (TH2) immunity, which enhances immune responses to antigens or allergens through adaptive and innate immune mechanisms, leading to the development and/or progression of allergic diseases. Viral infections can also trigger TSLP production in epithelial cells. TSLP supports the survival of cytotoxic T cells, both directly and indirectly, by activating dendritic cells (DCs).

(Data source: Ebina-Shibuya R, et al. Nat Rev Immunol. 2023)
TSLP plays an important role in the control and development of various cancers, including solid tumors such as breast cancer and leukemia. TSLP has both pro-tumor and anti-tumor effects. TSLP primarily promotes tumors by establishing T helper 2 (TH2)-type inflammation in the tumor microenvironment, primarily through activation of dendritic cells (DCs).

(Data source: Ebina-Shibuya R, et al. Nat Rev Immunol. 2023)
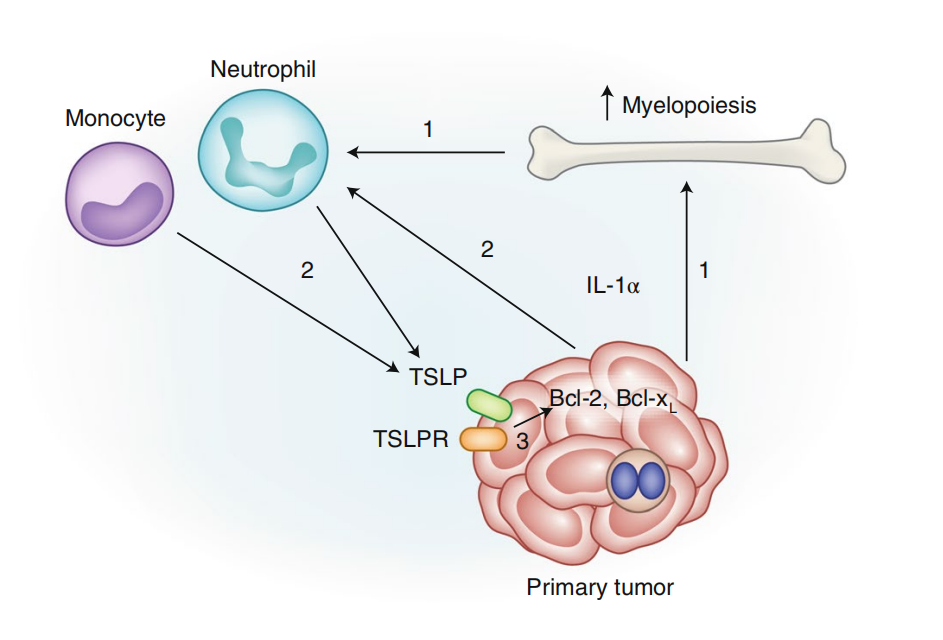
In breast cancer, IL-1α from tumor cells leads to increased myeloid hematopoiesis in the bone marrow, which increases the number of circulating neutrophils and inflammatory monocytes. IL-1α recruits neutrophils and monocytes into the tumor and induces TSLP expression. Tumor cells respond to TSLP from infiltrating myeloid cells with increased expression of Bcl-2 and Bcl-xL, which contributes to tumor cell survival.
(Data source: Corren J, et al. Nat Immunol. 2019)
However, other studies have shown that TSLP may have tumor-suppressive activity in breast cancer and cutaneous T-cell lymphoma. Thus, in a range of malignancies, TSLP has been linked to either promoting or reducing cancer severity, suggesting that this cytokine has context-dependent effects in malignant diseases.
Targeted therapy for TSLP
In 2021, the US FDA approved tezepelumab for the treatment of severe asthma. It is a human monoclonal antibody against TSLP that can block its binding to TSLPR and its biological effects, reduce eosinophil inflammation and AHR, and reduce the exacerbation of asthma patients.
