Background
Recent advances in the study of CLDN18.2 (Claudin18.2) in gastric and pancreatic cancers have made CLDN18.2 a promising therapeutic target for these two cancers. CLDN18.2 is a tight junction protein that is selectively expressed in cancer cells and rarely expressed in normal tissues, making it an attractive candidate for targeted therapy. Therapies such as monoclonal antibodies (such as Zolbetuximab ), bispecific antibodies, and antibody-drug conjugates have shown great potential in improving clinical outcomes. Early clinical trials have demonstrated strong anti-tumor activity, particularly in combination with chemotherapy and immunotherapy regimens. However, challenges remain, such as patient selection, resistance mechanisms, and toxicity management.
On December 1, 2024, Alireza Tojjari's team published an article titled "Emerging targets in gastric and pancreatic cancer: Focus on CLDN18.2" in Cancer Lett. The article reviewed the current application, clinical progress and future development direction of CLDN18.2 in the treatment of gastric cancer and pancreatic cancer.

Expression and function of CLDN18.2
CLDN18.2 , a Claudin family protein, is exclusively expressed in differentiated gastric epithelial cells and stem cells. It is abnormally overexpressed in the development and progression of various primary malignant tumors. Studies have shown that PMA, c-jun, epidermal growth factor (EGF), and RAS can upregulate CLDN18 expression, while IL-1β, STE20/SPS1, and SPAK can inhibit CLDN18 expression.
normal tissue, CLDN18.2, as part of the tight junction proteins, primarily functions to maintain barrier integrity and selective permeability between gastric epithelial cells. The shielding effect of the tissue adhesion complex protects CLDN18.2 from external factors, such as digestive enzymes, ensuring normal function of the gastric mucosa. During malignant transformation, cell adhesion is disrupted, and the CLDN18.2 epitope is exposed on the surface of gastric and GEJ ( G/GEJ ) adenocarcinoma cells, making it a promising target. CLDN18.2 is expressed in approximately 70% of gastric cancers and 60% of pancreatic cancers, making it an attractive target for therapeutic intervention.

(Data source: Nakayama I, et al. Nat Rev Clin Oncol. 2024)
The structure of CLDN18.2
CLDN18.2 is a cell surface protein encoding 264 amino acids with a molecular weight of approximately 27 kDa. It consists of a short cytoplasmic segment at the N-terminus, two extracellular loops formed by four transmembrane domains, and a C-terminal cytoplasmic tail. The larger extracellular loop plays a role in regulating the transcellular movement of ions. The smaller extracellular loop binds to CLDN18.2 molecules expressed on the surface of neighboring cells, forming a selective permeability barrier that enables tissue-specific permeability and thus supports the polarity of gastric epithelial cells.
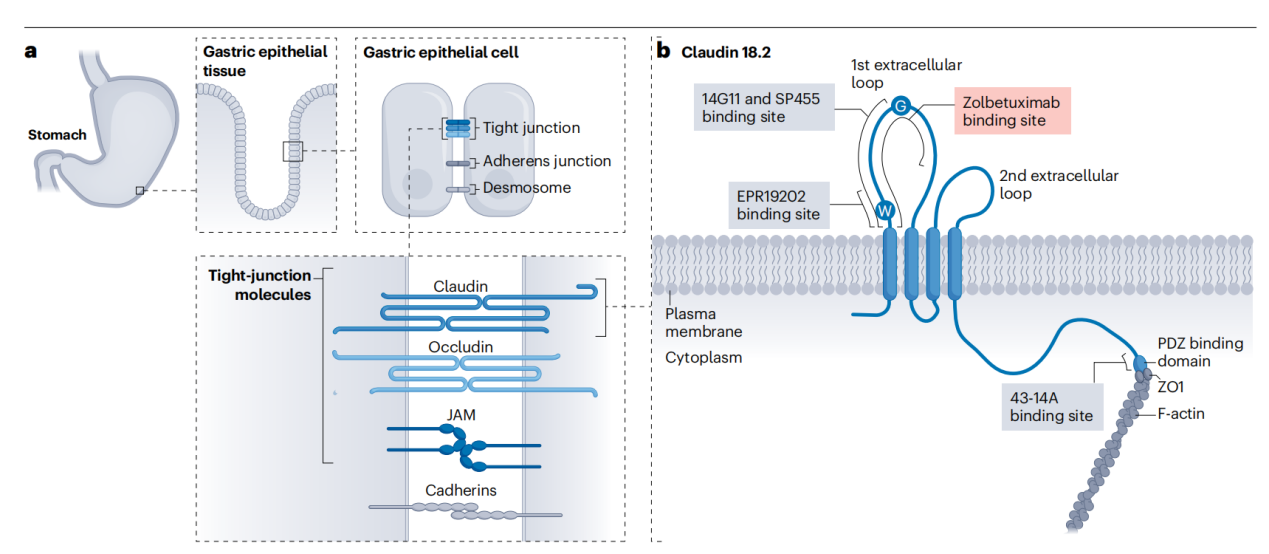
(Data source: Nakayama I, et al. Nat Rev Clin Oncol. 2024)
Targeted therapy for CLDN18.2
CLDN18.2 is selectively expressed in gastric cancer cells and less expressed in normal tissues, making it an ideal target for targeted therapy. The main methods of targeted therapy include monoclonal antibodies, antibody-drug conjugates, chimeric antigen receptor ( CAR) T cell therapy, and bispecific antibodies, each of which has different mechanisms.

Monoclonal antibodies
targeting CLDN18.2 (CLDN18.2) represent a major breakthrough in the treatment of gastric and pancreatic cancer. Among these therapies, zolbetuximab remains the most advanced in the clinic, along with AB011, MIL93, and osemitamab.
Zolbetuximab (IMAB362), a chimeric monoclonal antibody targeting CLDN18.2, has been shown to prolong progression-free survival (PFS) and overall survival (OS) in the SPOTLIGHT trial. However, a significant limitation is the lack of improvement in objective response rate (ORR), suggesting that its primary benefit lies in disease stabilization rather than inducing tumor shrinkage . The lack of improvement in ORR may be due to the low immune infiltration of some tumors and the inhibitory effects of regulatory T cells (Tregs) and cytokines such as TGF-β. Combining zolbetuximab with immune checkpoint inhibitors may enhance tumor infiltration and reverse immunosuppression. Modifying the Fc region to enhance ADCC efficacy in low-infiltration tumors may further optimize results.

(Data source: Nakayama I, et al. Nat Rev Clin Oncol. 2024)
Antibody-drug conjugates (ADCs)
CMG901 can deliver single MMAE to CLDN18.2-positive tumor cells and induce apoptosis by inhibiting microtubule formation.
Early trials in gastroesophageal junction (G/GEJ) cancer have shown promising antitumor effects and durable responses, although gastrointestinal side effects such as nausea and diarrhea have been noted.
SYSA1801, another MMAE-based ADC targeting CLDN18.2, has demonstrated potent antitumor activity in preclinical studies, particularly in drug-resistant or refractory solid tumors. An ongoing Phase I clinical trial has shown promise in the treatment of pancreatic cancer, but further clinical data are needed. Toxicity appears to be relatively mild, but a comprehensive safety assessment has yet to be conducted.
RC118 also utilizes an MMAE payload to target tumors expressing CLDN18.2 and has demonstrated potent antitumor effects in a variety of solid tumors, including pancreatic cancer.
Chimeric antigen receptor (CAR) T cell therapy
targeting CLDN18.2 has demonstrated high objective response rates (ORRs) in early trials, but achieving long-term progression-free survival (PFS) and duration of response (DOR) has been difficult in solid tumors such as gastric and pancreatic cancer. This may be due to the immunosuppressive properties of the tumor microenvironment. Incorporating IL-15 into CAR-T cell development can enhance T cell survival and proliferation, helping CAR-T cells persist longer in the immunosuppressive tumor microenvironment of solid tumors such as pancreatic cancer.
Bispecific antibodies
Bispecific antibodies (BsAbs) enhance the body's immune response to tumors by targeting CLDN18.2 together with immune effector cells or checkpoint molecules.
QLS31905 targets CLDN18.2 and CD3, directing T cells to tumor sites to induce cytotoxicity. Preclinical studies have shown promising efficacy in CLDN18.2-positive tumors, particularly in gastric cancer models, while early safety data suggest mild side effects.
PT886 is a bispecific antibody targeting CLDN18.2 and CD47. It promotes phagocytosis of tumor cells by blocking CD47, a "don't eat me" signal used by cancer cells to evade immune clearance. Preclinical studies have shown that PT886 has significant anti-tumor effects even at low doses and has the potential to completely eliminate tumors in some models.
Q-1802 simultaneously targets CLDN18.2 and PD-L1, combining immune checkpoint inhibition with direct tumor targeting. Early trials have shown that Q-1802 has promising anti-tumor activity in gastric adenocarcinoma and pancreatic cancer, particularly in relapsed or refractory tumors.

(Data source: Nakayama I, et al. Nat Rev Clin Oncol. 2024)
Studies targeting CLDN18.2-specific T cell immunotherapy have shown that T cells expanded with the CLDN18.2 peptide exhibit potent anti-tumor activity and enhanced cytokine secretion in vitro. These findings highlight the selective expression of CLDN18.2 in gastric cancer cells and its immunogenic potential, making it an ideal target for personalized immunotherapy.
Application of anti-CLDN18.2 combination therapy in tumor research
Recent advances in oncology research suggest that combining anti-CLDN18.2 therapy with immunotherapy, particularly ICIs, may offer a synergistic approach to enhance efficacy. Monoclonal antibodies targeting CLDN18.2, such as zobetuximab, direct the immune system to CLDN18.2-expressing tumor cells, leading to antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity. On the other hand, ICIs such as pembrolizumab and nivolumab work by removing inhibitory signals on immune cells, particularly T cells, enabling them to more effectively recognize and attack tumor cells. The combination of these two therapeutic modalities—CLDN18.2-targeted therapy and immune checkpoint blockade—creates a favorable therapeutic environment. The targeted specificity of CLDN18.2 antibodies enhances tumor killing, while ICIs enhance the immune system's ability to sustain anti-tumor responses.

Resistance mechanisms to CLDN18.2-targeted therapy
The main mechanisms of resistance to CLDN18.2-targeted therapies include antigen loss or heterogeneity, an immunosuppressive tumor microenvironment, activation of alternative signaling pathways, and immune evasion by tumor-associated macrophages. Combining CLDN18.2-targeted therapies with immune checkpoint inhibitors can amplify the immune response to offset antigen loss and reverse T cell exhaustion . CLDN18.2 -targeted drugs can be combined with pathway inhibitors (such as MEK or ERK inhibitors) to block downstream signaling cascades. Dual CAR-T approaches targeting CLDN18.2 and other antigens critical for tumor survival are also possible.
Summary and Outlook
targeting CLDN18.2 primarily include monoclonal antibodies, antibody-drug conjugates, CAR-T cell therapy, and bispecific antibodies. These have demonstrated strong anti-tumor activity against CLDN18.2-positive tumors. However, resistance to CLDN18.2 therapy remains a significant obstacle. Through innovative combination therapies, resistance management, and precision medicine approaches, the future of CLDN18.2 - targeted therapies will address key challenges in the treatment of gastric and pancreatic cancer. Future research should continue to unravel the complex role of CLDN18.2 in cancer progression, aiming to identify predictive biomarkers and optimize treatment options. These targeted therapies hold great potential to significantly improve clinical outcomes, marking a new era in the treatment of gastric and pancreatic cancers.
