Activin receptor type 1(ACVR1), also known as ALK2, is a type I receptor for bone morphogenetic protein (BMP) that is involved in multiple biological processes, including the development and regulation of the skeletal, cardiac, cartilage, nervous, and reproductive systems . Gain-of-function mutations in ACVR1, such as R206H, cause fibrodysplasia ossificans progressiva (FOP).
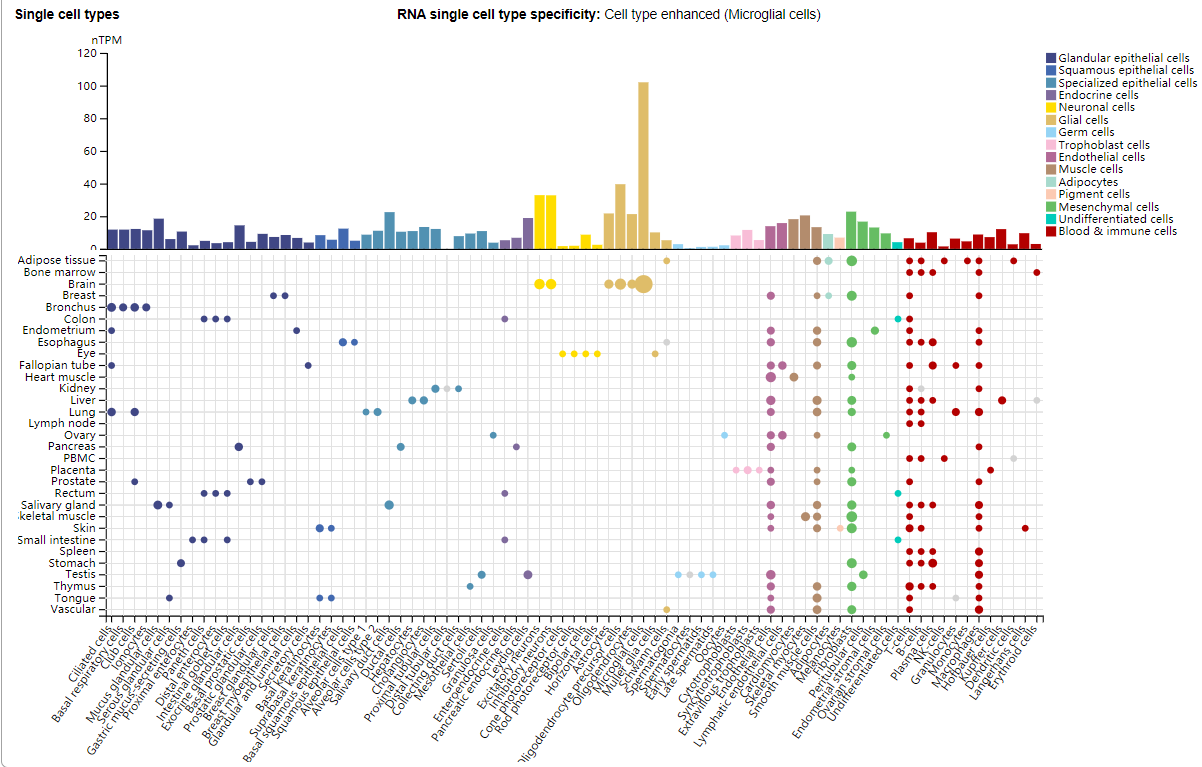
ACVR1 expression distribution
ACVR1 is expressed in microglia, oligodendrocyte precursor cells, excitatory neurons, inhibitory neurons, and to a lesser extent in glandular epithelial cells, squamous epithelial cells, specialized epithelial cells, endocrine cells, mesenchymal cells, and immune cells.
(Data source: Uniprot)
Structure of ACVR1
The human activin A receptor type I (ACVR1) gene is located on chromosome 2q23-q24. The ACVR1 protein is a member of the BMP/TGFβ receptor family and consists of an extracellular N-terminal ligand-binding domain, a transmembrane (TM) domain, an intracellular glycine-serine (GS)-rich domain, and a protein kinase (PK) domain. A loop within the helix-loop-helix of the GS domain contains key residues for ACVR1 activation upon phosphorylation. As a type I receptor, ACVR1 forms a heterotetrameric receptor complex with the type II receptors BMPR2, ACVR2A, and ACVR2B. This complex consists of two type I and two type II receptors. Upon ligand binding to the heteromeric complex, the type II receptor transphosphorylates the GS domain of the type I receptor. Consequently, the kinase domain of the type I receptor is activated, subsequently phosphorylating SMAD1/5/8 proteins, transducing the signal.

(Data source: Valer JA, et al. Cells. 2019)
Signal transduction regulation of ACVR1
ACVR1 can trigger classical SMAD1/5/8 or non-SMAD signaling pathways.
Classical SMAD signaling pathway
Upon binding of BMP ligands (such as BMP6/7/9) and activins (such as activin A/B ) , ACVR1 forms a complex with type II receptors, phosphorylating SMAD1/5/8. This complex then binds to SMAD4 and translocates to the nucleus, regulating target genes such as Runx2. This pathway directs cartilage formation and bone development. Mutations (such as R206H) lead to ligand-independent activation and SMAD hyperphosphorylation, driving heterotopic ossification.
Non-SMAD signaling pathways
ACVR1 transmits signals through the MAPK (p38/ERK/JNK), PI3K/AKT/mTOR, and RhoA pathways, activating transcription factors such as RUNX2 and regulating the cytoskeleton. These pathways cross-talk with mechanotransduction, and upon mutation, synergistically amplify osteogenic signals. In particular, the RhoA-YAP/TAZ axis misjudges the microenvironmental stiffness and promotes pathological differentiation.

(Data source: Mejias Rivera L, et al. Biomedicines. 2024)
ACVR1-targeted therapy
Therapeutic strategies targeting ACVR1 primarily target abnormal signaling pathway activation caused by its mutations (e.g., in diseases such as FOP and diffuse intracellular pontine glioma/DIPG). Activin A-blocking antibodies, such as garetosmab, inhibit activin A-mediated signaling. ACVR1 inhibitors, including fidrisertib and zilurgisertib, inhibit the activity of mutant ACVR1 receptors.

(Data source: Anwar S, et al. Genes (Basel). 2023)
Garetosmab, a fully human monoclonal antibody developed by Regeneron, specifically targets and inhibits activin A. The drug inhibits abnormal signaling by blocking the binding of activin A to ACVR1 and is used to treat fibrodysplasia ossificans progressiva (FOP). Although clinical trials were suspended in 2020 due to a patient safety incident, Phase III clinical trials were restarted in 2022, and an IND application was submitted in China. Regeneron announced its ongoing investigational combination therapy for the treatment of obesity: semaglutide (a GLP-1 receptor agonist) and trevogrumab (anti-GDF8/anti-myostatin), with or without garetosmab.
