Background
Immunocytokines utilize targeted antibodies to concentrate cytokines at tumor sites and have shown potential advantages, such as prolonged cytokine half-life, reduced adverse reactions, and synergistic antitumor efficacy of antibody and cytokine components.

On August 2, 2024, Acta pharmaceutica Sinica. B published an article titled "Advancements and challenges in immunocytokines: A new arsenal against cancer." This review focuses on the molecular structure design of cytokines and explores potential strategies that can promote the development of next-generation immunocytokines, such as cytokine engineering and prodrug design. It emphasizes the direction and focus of ongoing efforts to improve safety while maximizing efficacy.

Overview of Immune Cytokines
Cytokines play a crucial role in immune regulation, modulating the tumor microenvironment and exhibiting potent anti-tumor effects. However, the clinical application of cytokines has long been limited by their short half-life and off-target systemic toxicity, leading to a "cytokine sink" effect. In 2024, N-803 (an IL-15 superagonist) was approved, becoming the third cytokine to be marketed. However, it is indicated for the treatment of non-muscle invasive bladder cancer (NMIBC) in combination with intravesical BCG instillation, rather than systemic administration (NCT03022825).

Immunocytokines (antibody-cytokine fusion proteins) use antibodies to selectively deliver cytokines to tumors, thereby extending their half-life and reducing toxicity. Many immunocytokines have entered clinical trials. For example, KD033, a fusion of IL-15 and PD-L1, demonstrated good tolerability in a Phase I clinical trial. This trend reflects the growing trend of immunocytokine research toward targeting specific immune checkpoints, such as PD-1/PD-L1.

Common immune cytokines
IL-2
IL-2 has been used to produce various immune cytokines targeting alternatively spliced extra domain A (EDA) or extra domain B (EDB) of fibronectin, carcinoembryonic antigen (CEA), fibroblast activation protein (FAP), PD-1, and others. Currently, a variety of IL-2-derived immune cytokines are in preclinical and clinical development.
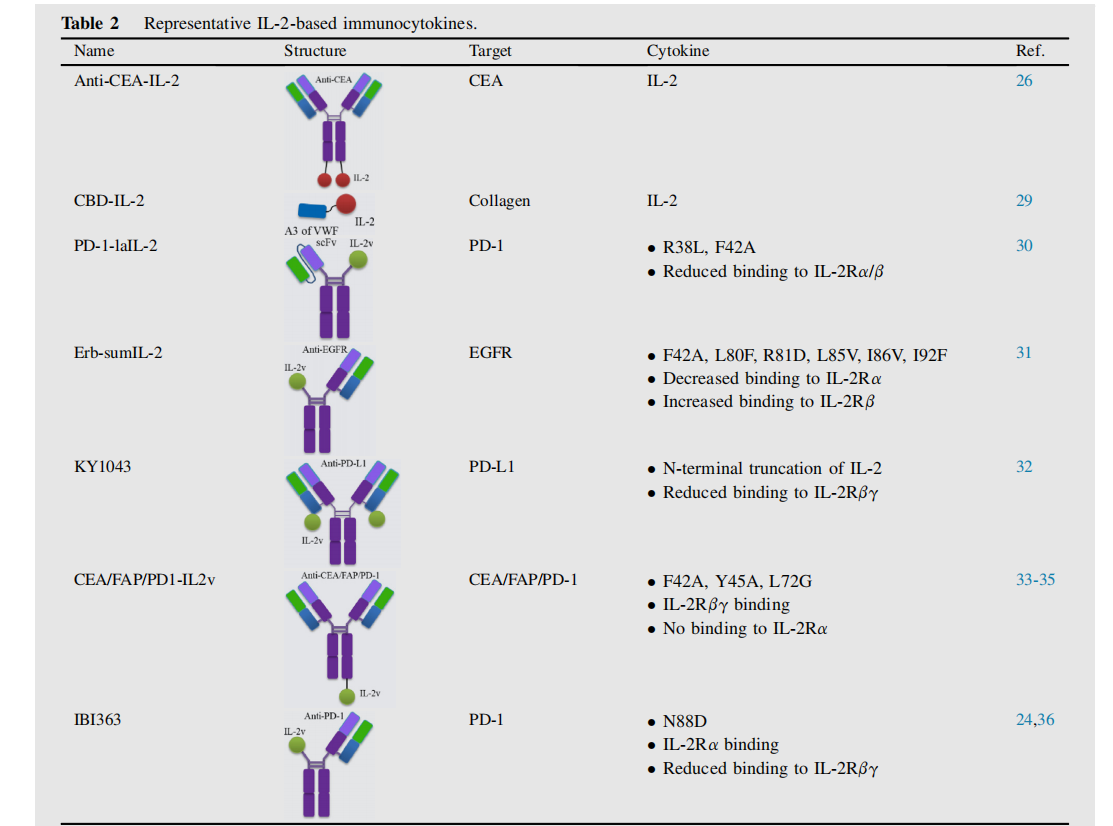
Dimeric immune cytokines anti-CEA-IL-2, IL-2 fused with CBD (CBD-IL-2), their anti-tumor effects depend on CD8+T cells in the tumor; a new low-affinity IL2 immune cytokine PD-1-laIL-2 fused with PD-1; E rb-sumIL2 and Her2-sumIL2 with asymmetric structures composed of hypermutated IL2 ( sumlL-2 ), and KY1043 formed by the fusion of PD-L1 and IL-2 mutants ; they all show stronger anti-tumor effects, and their anti-tumor effects depend on CD8+T cells.
Roche has developed a new class of immunocytokines based on engineered IL-2 variants (IL-2v) that retain affinity for IL-2Rβγ while abrogating binding to IL-2Rα. Examples include CEA-IL2v and FAP-IL2v, which exhibit enhanced tumor targeting and improved pharmacokinetics compared to wild-type IL-2.
Innovent has developed an anti-PD-1/IL-2 fusion protein (IBI363) that blocks the PD-1 checkpoint and cis-activates α-subunit-biased IL-2 to restore depleted TSTs. Studies have shown that compared with IL - 2R βγ-biased agonists, IL - 2R α-biased agonists significantly enhance tumor-specific CD8+ T cells and demonstrate superior anti-tumor efficacy. Furthermore, IL - 2R α-biased agonists exhibit stronger stimulation of peripheral regulatory T cells, resulting in a better safety profile than IL - 2R βγ-biased agonists.
IL-15
IL-15 plays a crucial role in the development, survival, and activation of NK cells and CD8+ T cells. It also contributes to the homeostatic proliferation of memory CD8+ T cells, promoting long-lasting immunity against tumors. IL-15 has been fused to various targeting antibodies or proteins, including the transforming growth factor β receptor extracellular domain (TGF-βRII-ECD), anti-PD-1, and anti-PD-L1.
N-809 is a bifunctional fusion protein consisting of an IL-15/IL-15Rα superagonist complex containing an IgG1 Fc domain fused to two single-chain anti-PD-L1 domains . Mechanistically, N-809's anti-tumor effects are mediated through NK cells and CD8+ T cells.
LH01 showed better anti-tumor efficacy and safety than the combination of anti-PD-L1 and non-targeted IL-15 superagonist and overcame resistance to anti-PD-L1 treatment.

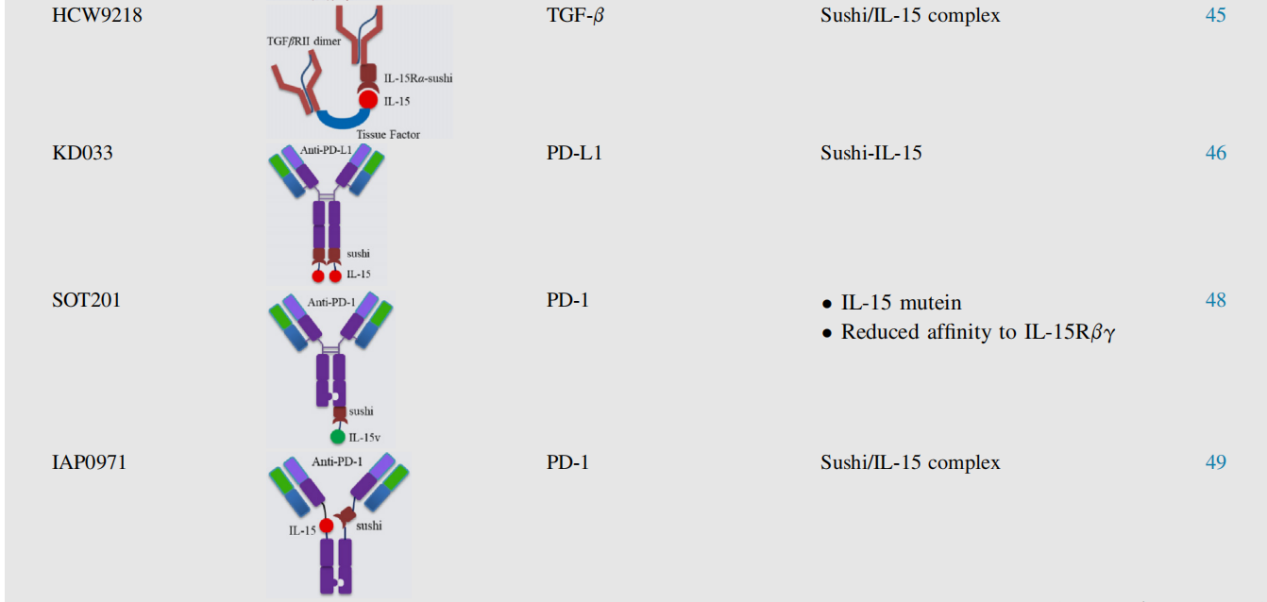
HCW9218 utilizes soluble tissue factor-based scaffold technology, combines TGF-βRII-ECD and IL-15Rα-sushi domain/IL-15 complex, and exhibits potent anti-tumor activity by promoting NK cell and CD8+ T cell infiltration into tumors.
KD033 consists of a fully human anti-PD-L1 linked to an IL-15Rα-sushi domain/IL-15 complex. KD033 activates both adaptive and innate immune cells within tumors, with its anti-tumor efficacy primarily dependent on CD8+ T cells rather than NK cells. Furthermore, when used in combination with anti-PD-1, KD033 exhibited synergistic anti-tumor activity. In a Phase I clinical trial, KD033 was well tolerated, with predictable and manageable adverse events.
SOT201 and IAP0971 rely on CD8+ T cells and NK cells. The safety, tolerability, and preliminary efficacy of IAP0971 are currently being evaluated in a Phase I/IIa clinical trial (NCT05396391) in patients with advanced malignancies.
Studies have found that NK cells can lyse tumors with minimal activation and are not restricted by MHC. IL-15 integrated with trispecific killer cell ligands (TriKEs) exhibits enhanced NK cell proliferation and activation.
Miller's team used a modified IL-15 crosslinker to construct a TriKE (GTB-3550) containing single-chain antibodies against NK cell CD16 and tumor cell CD33. This antibody can effectively restore defective NK cell function and induce specific NK cell proliferation. A phase 1 study demonstrated the safety of GTB-3550 TriKE in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome, robust expansion of endogenous NK cells, and active clinical signals (NCT03214666).

IL-12
IL-12 plays a crucial role in promoting the differentiation and proliferation of Th1 cells, reactivating and promoting the survival of CD4+ T cells, and enhancing the cytotoxicity of CD8+ T cells and NK cells. However, the clinical application of IL-12 faces dose-limiting toxicity. In a Phase I clinical trial, the maximum tolerated dose of rhIL-12 was only 500 ng/kg. Fusion of IL-12 with antibodies to construct immune cytokines could improve this problem.
IL12-F8-F8 is an immunocytokine based on the sequential fusion of IL-12 as a single polypeptide with two F8 antibodies in a single-chain antibody fragment (ScFv) format. It targets the alternatively spliced fibronectin EDA. Natural killer (NK) cells are primarily involved in the anti-tumor effects mediated by IL12-F8-F8.
Wittrup's team pioneered a method to enhance the retention of injected cytokine fusion proteins by leveraging the abundant collagen in tumors. Compared with non-anchored IL-12, collagen-anchored IL-12 administered intratumorally has a longer retention time and effectively eliminates the toxicity of systemic exposure . Combining it with cancer vaccines, CAR-T cell therapy, or neoadjuvant checkpoint blockade can improve anti-cancer efficacy without exacerbating toxicity. Furthermore, this local treatment can initiate protective and systemic CD8+ T cell responses, resulting in abscopal effects that can cure tumors that have not been injected with cytokines.
The CBD-IL-12 developed by Hubbell's team showed enhanced anti-tumor activity and reduced systemic toxicity. CBD-IL-12 can synergize with checkpoint inhibitors and trigger antigen-specific immune responses.
L19-IL-12 consists of IL-12 and a single-chain variable fragment (scFv) that selectively binds to the alternatively spliced EDB domain of fibronectin. L19-IL-12 can increase the infiltration and activation of CD4+ T cells, CD8+ T cells, and NK cells in mouse glioma models. Philogen has initiated a Phase I clinical trial (NCT04471987) in patients with advanced solid tumors whose disease has progressed after immune checkpoint blockade therapy.

IL21
IL-21 has shown promising anti-tumor activity in clinical trials by enhancing the effector functions of NK cells and T cells, and regulating B cell differentiation and survival.
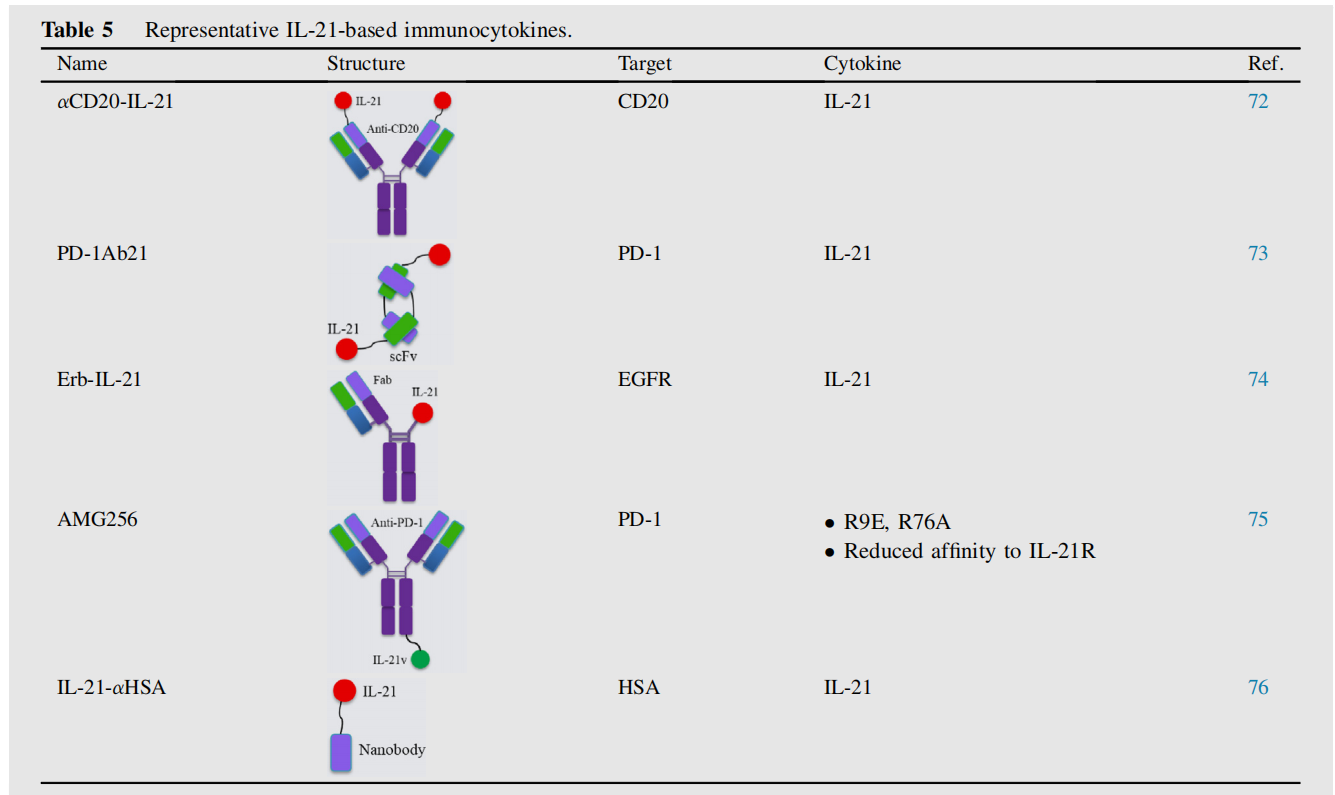
αCD20-IL-21, a human IL-21 fusion protein fused to the NH2 terminus of anti-CD20, directly induces apoptosis in IL-21-resistant B-cell lymphoma cells. It also enhances NK cell activation, effector function, and IFN-γ production, leading to greater ADCC. αCD20-IL-21 treatment demonstrated improved tumor control in rituximab-resistant A20-huCD20 tumors.
Amgen has reported a novel immunocytokine fused to an anti- PD -1 antibody and an IL-21 cytokine mutein. AMG256, fused to IL-21, demonstrated significant efficacy in a refractory humanized mouse model. A Phase I clinical trial (NCT04362748) has been established to evaluate the safety and tolerability of AMG256.
IL -21-αHSA (JS014) is an engineered, long-acting IL-21 fused to a nanobody targeting human serum albumin (HSA) . This design significantly prolongs the half-life of IL-21 and increases its exposure in cynomolgus monkeys. When combined with PD-1 and T cell immunoreceptor Ig and ITIM domain (TIGIT) blockers, IL-21-αHSA not only enhances anti-tumor responses but also protects against tumor rechallenge, suggesting the establishment of long-term anti-tumor immunity.
IFNα
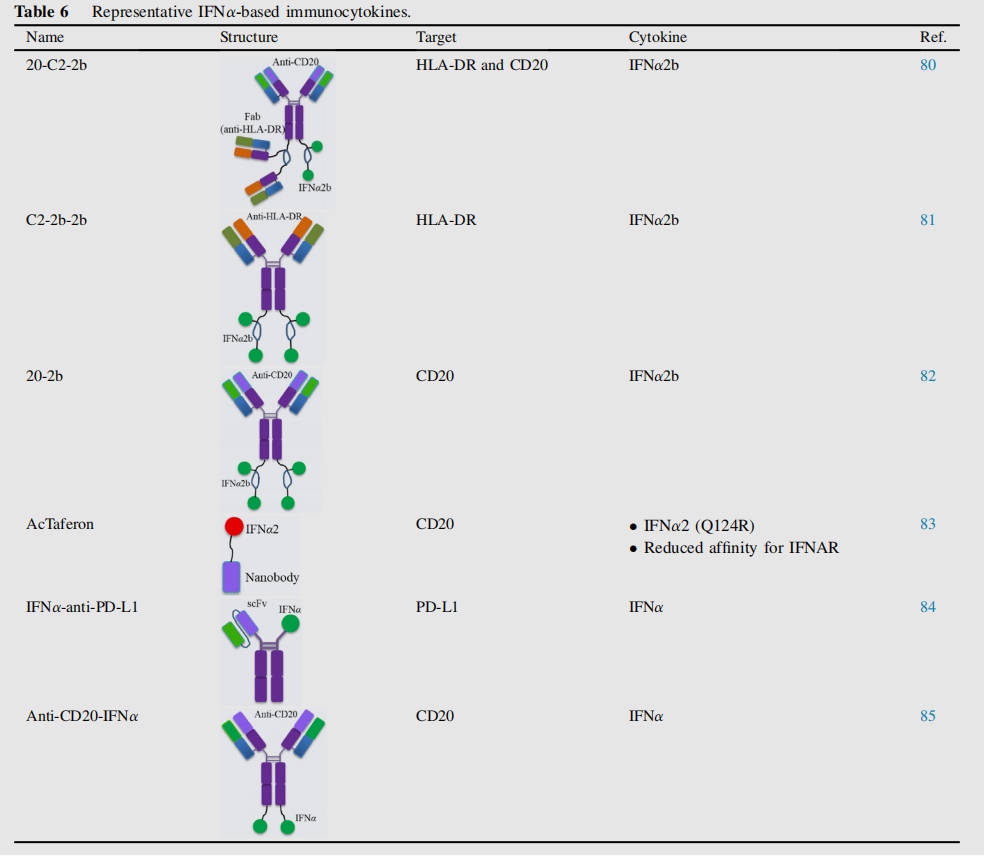
IFNα, a member of the type I interferon family, plays a key role in regulating genes that influence tumor growth, proliferation, migration, apoptosis, and differentiation. The clinical application of IFN-α has long been limited by side effects and a short half-life. IFNα-based immune cytokines, such as 20-C2-2b, C2-2b-2b, and 20-2b, primarily target hematologic malignancies.
In addition to their anti-tumor effects, IFNs are also powerful inducers of PD-L1 expression, thereby suppressing T cell responses to tumors. By designing IFNα -anti-PD-L1 to induce a feed-forward response, this approach synergistically overcomes resistance to type I IFN and checkpoint blockade therapies while minimizing side effects in advanced tumors.
IGN002 is an anti- CD20 -IFNα fusion protein. Anti-CD20-IFNα-mediated tumor eradication requires expression of type I IFN receptors on the tumor cell surface, while optimal tumor suppression requires CD20 targeting. 85 A Phase I study evaluating the safety and efficacy of IGN002 in patients with refractory non-Hodgkin lymphoma is ongoing.
Novel immune cytokines
IL-10
The only reported IL-10-based immunocytokine is CmAb-(IL10) 2 , which features an IL-10 dimer replacing one arm of cetuximab. It has superior anti-tumor efficacy and toxicity compared to both non-targeted IL-10 and cetuximab. Mechanistically, CmAb-(IL10) 2 inhibits IFN-γ-induced intratumoral CD8+ T cell apoptosis through IL-10 receptor signaling on dendritic cells. These findings highlight the significant potential of IL -10-based immunocytokines in cancer therapy.

IL-18
IL-18 has significant anti-tumor potential , but the presence of IL-18BP (IL-18 binding protein) hinders the binding of IL-18 to its receptor, thereby acting as a secretory immune checkpoint in the TME, thereby limiting its therapeutic efficacy. The IL-18 mutant DR-18 exerts potent anti-tumor effects by promoting the development of multifunctional effector CD8+ T cells, reducing T cell exhaustion, and expanding the pool of stem TCF-1+ precursor CD8+ T cells.

(Data source: Zhou T, et al. Nature. 2020)
IL24
IL-24 has the ability to inhibit tumor invasion, metastasis, and tumor stem cell stemness. Clinical studies have shown that reduced IL-24 expression is associated with tumor progression and poor prognosis. IL-24's anti-tumor effects are also attributed to its ability to effectively reduce vascular endothelial growth factor levels, stimulate CD4+/CD8+ T cells, and transform the TME into a more immunostimulatory environment. The construction of antibody-cytokine fusion proteins targeting IL-24 at tumor sites has significant potential in overcoming drug resistance and immune escape.
TNF-α
TNF-α was originally considered to be cytotoxic, but is now also recognized to have immunosuppressive functions, promoting the recruitment and/or activity of T regs, regulatory B lymphocytes (Bregs), and MDSCs.98 L19 -TNF is one of the earliest cytokines studied in this field.

Next-generation immune cytokines
Immunocytokines are hampered by the " cytokine sink " effect, whereby they are captured by circulating cognate receptors before reaching target tumor sites. Two strategies are currently underway to address this "cytokine sink" and improve tumor targeting: using cytokine mutants with reduced receptor affinity or creating conditionally activated immunocytokine prodrugs.
Immune cytokine fusion with cytokines of reduced potency
Reducing the affinity of cytokines for their cognate receptors can mitigate the " cytokine pull " effect, thereby extending their half-life and enabling antibody-directed tumor accumulation. Furthermore, due to the extended half-life, reduced bioactivity can increase the amount of drug at the tumor site, allowing the use of higher doses of immunocytokines. Mutations in cytokines such as IL-2, IL-15, and IFNα have been shown to enhance the drug delivery of these immunocytokines.

Conditionally activated immunocytokine prodrugs
Prodrug-based strategies offer a potential approach to improving the safety of immunocytokines while maintaining antitumor activity. Utilizing tumor-associated proteases is a promising approach for achieving localized cytokine activation in tumors. Various masking domains, such as native cytokine receptors, antibody fragments, peptides, and polyethylene glycol ( PEG ), have been used to mask cytokines. In healthy tissues, cytokines are shielded by masking groups; in tumors, they are cleaved by tumor-associated proteases, redox reactions, or acidic pH, releasing the immunostimulatory cytokine.

Summarize
For "matching" antibodies and cytokines is that immunocytokines should exhibit superior antitumor activity and safety compared to combined administration of antibodies and cytokines. Immunocytokine safety can be improved by modifying cytokine receptors with low natural affinity and using prodrug technology. Combining these two strategies may offer a combination approach that optimizes safety, efficacy, and targeted activation of immunocytokine pathways. Immunocytokines have the potential to be used in combination with other therapeutic approaches, including antibodies, chemotherapy, radiotherapy, and adoptive cell transfer (ACT). Combining various cytokine products with different cytokine payloads can produce synergistic antitumor effects, necessitating optimization of the combined levels of different cytokines.
