Tissue factor pathway inhibitor ( TFPI ) is an alternatively spliced anticoagulant protein that primarily inhibits the initial stage of coagulation, preceding thrombin generation. Tissue factor (TF) is considered one of the most potent procoagulants in the body, initiating the coagulation process for both physiological hemostasis and pathological thrombosis. TFPI's effects are restricted to cells expressing TF and sites of injury, making it a key regulator of bleeding in hemophilia.
Expression distribution of TFPI
TFPI is mainly expressed in the extravillous trophoblast, lymphatic endothelial cells, and syncytiotrophoblast, and is involved in the balance of blood coagulation and anticoagulation.
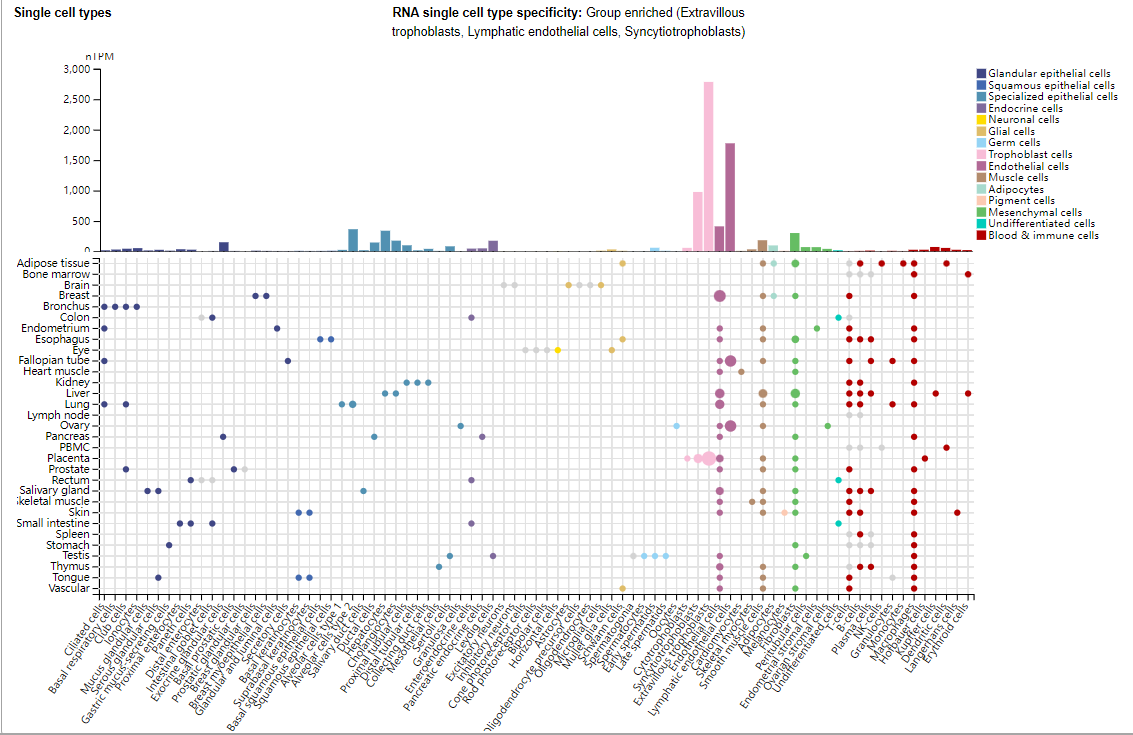
(Data source: Uniprot)
The structure of TFPI and its isomers
TFPI is a single-chain glycoprotein belonging to the Kunitz-type serine protease inhibitor family. It consists of 304 amino acids and its structural feature is the presence of three tandem Kunitz-type inhibitory functional domains, which are called K1, K2, and K3 from the N-terminus to the C-terminus.

(Data source: uniprot)
TFPI is produced through distinct cleavage events into two primary isoforms: TFPIα and TFPIβ. TFPIα and TFPIβ possess distinct C-termini. Variation in these C-termini targets each isoform to distinct locations within the vascular system and alters its anticoagulant activity. These two major isoforms share an identical N-terminus, consisting of an acidic amino acid stretch and two Kunitz-type serine protease inhibitory domains. These two Kunitz domains (K1 and K2) inhibit the tissue factor-factor VIIa (TF-FVIIa) catalytic complex in a factor Xa (FXa)-dependent manner.

(Data source Mast AE, et al. J Thromb Haemost. 2022)
Mechanisms of TFPI inhibition of coagulation
TFPI directly binds to the active site of FXa via its Kunitz 2 domain (K2), which underlies its anticoagulant activity. TFPI also directly binds to the active site of FVIIa via its Kunitz 1 domain (K1), but this inhibition requires the presence of FXa. TFPI can simultaneously inhibit the ternary complex formed by TF, FVIIa, and FXa, preventing TF-FVIIa-mediated prothrombin activation.
TFPIα, the only TFPI isoform present in platelets, interacts with the B domain of FVIIa via its C-terminus, inhibiting the formation of early prothrombinase. TFPIα inhibits prothrombinase by binding to the FXa active site via its K2 domain and interacting with FVIIa via its C-terminus.
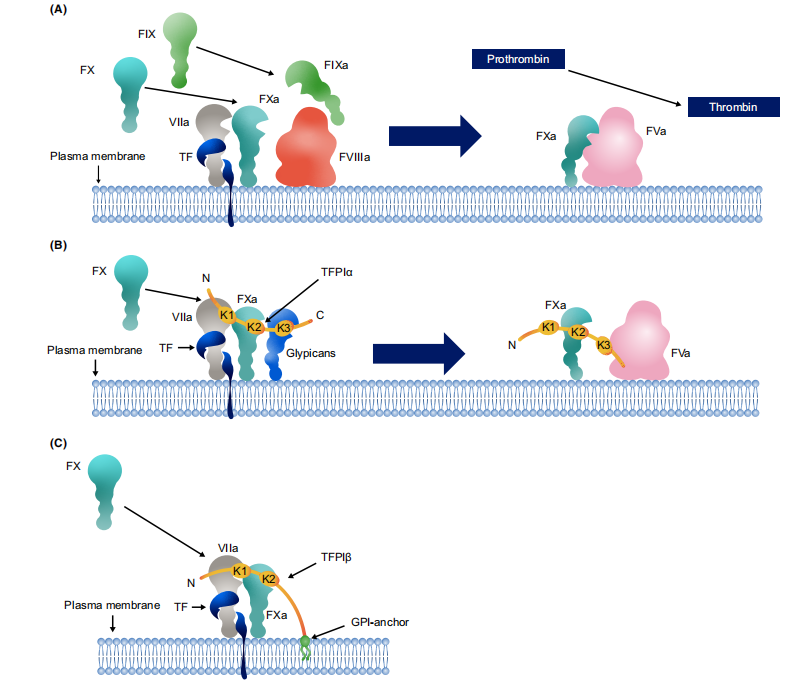
(Data source: Mast AE, et al. J Thromb Haemost. 2022)
Signal transduction and interaction between TFPI and TF:
TF is a key initiator of the extrinsic coagulation pathway. It binds to factor VIIa to form the TF-FVIIa complex, which in turn activates factor X (FX) and factor IX, initiating the coagulation cascade. TF also interacts with protease-activated receptors (PARs), particularly PAR2. TF-FVIIa-FXa complex activates PAR2 in the presence of endothelial protein C receptor (EPCR), contributing to inflammatory responses and signaling in vascular endothelial cells. TFPI can regulate cell signaling through interactions with cell surface receptors. For example, its K3 domain binds to glycosylphosphatidylinositol (GPI)-anchored receptors, affecting the function of cell-surface TFPI. TFPI may also regulate cellular uptake and degradation of FXa through interactions with receptors such as low-density lipoprotein receptor-related protein (LRP), thereby affecting signaling.
In autoimmune diseases, TFPI's inhibitory effects may be disrupted, leading to activation of the TF-FVIIa-FXa complex and triggering pathological signaling. In tumor biology, TFPI may influence tumor cell migration and invasion by regulating the activity of the TF-FVIIa-FXa complex. Because TFPI can inhibit TF-mediated angiogenesis, it is a potential candidate for anti-angiogenic therapy. By inhibiting tumor angiogenesis, it can suppress tumor growth and metastasis.

(Data source Mast AE, et al. J Thromb Haemost. 2022)
TFPI-targeted therapy
Currently approved antibodies targeting TFPI include Marstacimab and Concizumab . Marstacimab is a subcutaneously injected human monoclonal immunoglobulin G1 antibody against tissue factor pathway inhibitor (TFPI) developed by Pfizer for the treatment of hemophilia A and B.
Concizumab ( Alhemo™ ) is a subcutaneously administered humanized monoclonal IgG4 antibody against tissue factor pathway inhibitor ( TFPI ) that binds to the Kunitz-2 domain of TFPI and prevents TFPI from binding to activated Factor X. Concizumab was developed by Novo Nordisk for the treatment of hemophilia A and B with or without inhibitors.
Armocibart is a monoclonal antibody targeting TFPI developed by Alphamab Oncology for the treatment of hemophilia A, hemophilia B,Von Willebrand disease. A clinical trial, NCT05421429, investigated the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of multiple subcutaneous doses of KN057 in participants with hemophilia A or B, with or without factor VIII (FVIII) or factor IX (FIX) inhibitors.
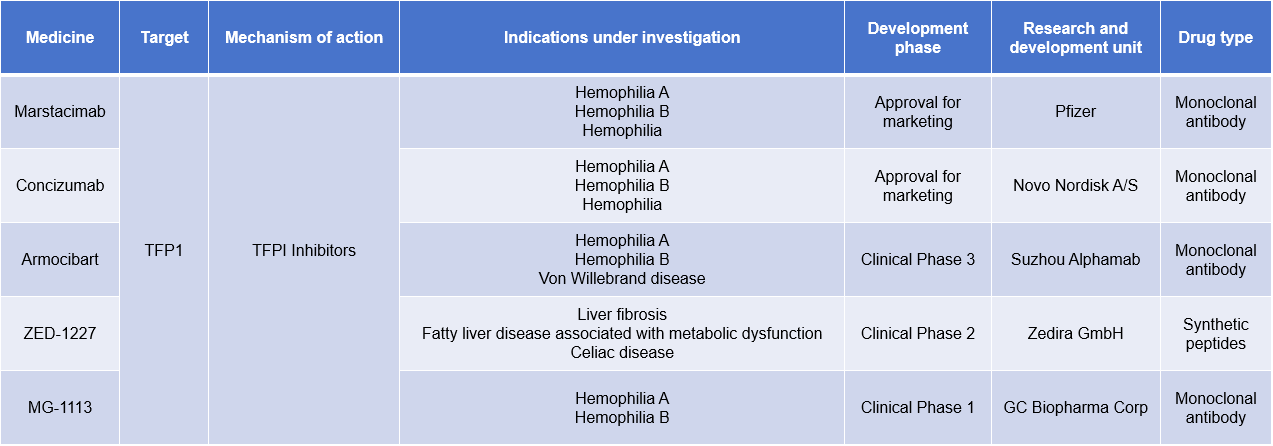
(Data source: New Drug Intelligence Database)
