Trophoblast cell surface antigen 2 (TROP2), also known as tumor-associated calcium signal sensor 2 (TACSTD2), is overexpressed in a range of solid tumors and significantly impacts tumor growth, invasion, and metastasis. Therefore, TROP2 has become an attractive prognostic biomarker and therapeutic target for solid tumors.
Expression distribution of TROP2
TROP2 is mainly expressed in basal respiratory cells, squamous epithelial cells, suprabasal keratinocytes, ionocytes, club cells, duct cells, basal squamous epithelial cells, and basal keratinocytes.
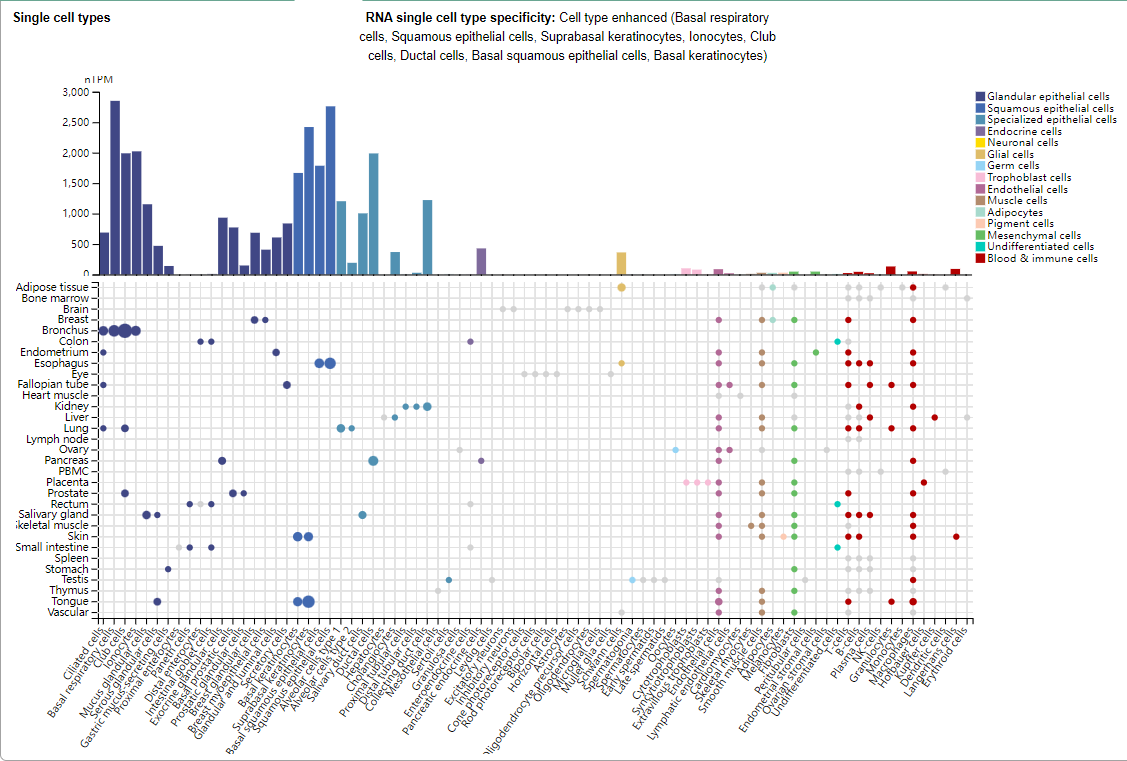
(Data source: Uniprot)
Structure of TROP2
TROP2 is a type I transmembrane glycoprotein composed of four domains, including a signal peptide (SP), an extracellular domain (ECD), a single transmembrane helix (TMD), and a cytoplasmic tail (ICD). The TROP2-ECD (H27-T274) contains three subdomains: a cysteine-rich domain (CRD), a thyroglobulin type 1 domain (TY), and a cysteine-poor domain (CPD).
TROP2-ECD can self- assemble into cis- or trans-dimers, or even tetramers. At the interface of cis-dimers, a complex network of hydrogen-bonding interactions is formed between the TY ring of one monomer and the βC sheet of the other. In contrast, in the case of trans- TROP2 -ECD dimers, the interaction is primarily mediated by the βC sheets of the two monomeric CPDs, which cover a relatively smaller surface area than in cis-dimers.

(Data source Liu X, et al. Theranostics. 2024)
TROP2 signaling pathway in tumors
TROP2 can activate multiple oncogenic signaling pathways. TROP2 activates the ERK1/2-MAPK axis, promoting malignant transformation and driving tumorigenesis. Furthermore, it regulates the Notch pathway, influencing stem cell function and potential tumor differentiation and progression. TROP2 also directly interacts with nuclear β-catenin to promote cell proliferation, a hallmark of cancer.

(Data source: Yao L, et al. Front Oncol. 2023)
TROP2-targeted therapy
Currently, the main treatments targeting TROP2 include ADC, antibodies, multispecific drugs, immunotherapy, cancer vaccines and small molecule inhibitors.

(Data source: Liu X, et al. Theranostics. 2024)
Antibody-drug conjugates (ADCs)
Preclinical studies have shown that Trodelvy ™ (sacituzumab govitecan), the first TROP2- targeting antibody-drug conjugate (ADC) , has significant and specific anti-tumor effects across various TROP2- positive tumor types. It has been shown to improve survival in metastatic breast cancer (mBC) and metastatic urothelial carcinoma (mUC). In a Phase I dual antibody-drug conjugate (DAD) trial, SG, combined with the nectin-4-directed ADC enfortumab vedotin (EV), achieved an impressive objective response rate (ORR) of 70% in patients with mUC.
Datopotamab deruxtecan ( Dato-DXd ). Dato-DXd is a novel TROP2 -directed ADC being developed by Daiichi Sankyo and AstraZeneca. It consists of a recombinant humanized anti-TROP2 IgG1 monoclonal antibody (mAb) conjugated to a topoisomerase I inhibitor (DXd) via a tetrapeptide-based linker that is linked to a cysteine residue at the datopotamab interchain disulfide bond. Dato-DXd has demonstrated significant potential and a manageable safety profile in the treatment of various TROP2-positive solid tumors, showing particular promise in non-small cell lung cancer (NSCLC). While the clinical potential of Dato-DXd is promising, its side effects require careful attention and management. Interstitial lung disease (ILD) is a significant risk associated with Dato-DXd, particularly in patients with NSCLC.
Although ADCs hold great promise for the treatment of TROP2-positive tumors, realizing this potential requires overcoming several key challenges, including intra- and intratumoral heterogeneity, TRAE risk, and drug resistance. Therefore, the development of safer and more effective targeted drugs that exploit the biological functions of TROP2 itself is needed.
Monoclonal antibodies
Due to the unique oligomeric properties of TROP2 structure, the large extracellular domain (ECD) makes it an ideal therapeutic target for targeted drugs aimed at blocking the oncogenic activity of TROP2.
RS7-3G11 (RS7), the antibody component of SG, was an extensively studied anti-TROP2 mAb in early studies. It recognizes a linear peptide Q237-Q252 within the extracellular domain (ECD) of TROP2, located within the cystine-deficient region (CPD) of TROP2. RS7 itself exhibits strong internalization activity but lacks therapeutic efficacy. Studies have shown that RS7 is unlikely to disrupt TROP2 oligomeric assembly. This may explain why the unbound form of RS7 has no inhibitory effect on TROP2-positive tumors.
The CPD region of TROP2 may contain multiple immunodominant epitopes, making it easy to obtain non-functional TROP2 antibodies. However, these epitopes are readily available in both tumor cells and normal cells, and TROP2-directed monoclonal antibodies targeting these epitopes may cause difficult-to-control extratumoral toxicity. Therefore, researchers have found that targeting tumor-specific epitopes on TROP2 seems more promising.

ARIUS Research used the FunctionFIRST platform to generate mAb AR47A6.4.2, which targets two linear epitopes within the TROP2-CPD (L179-H187 and Q252-Y260). AR47A6.4.2 inhibits TROP2 signaling and mediates downregulation of the mitogen-activated protein kinase (MAPK) pathway in response to serum stimulation. It also induces complement-dependent cytotoxicity (CDC) in human pancreatic cells. This dual MOA enables AR47A6.4.2 to inhibit tumor growth in human breast cancer (90%, p<0.00001), colon cancer (60%, p<0.001), and prostate cancer (60.9%, p<0.001) models.
Pr1E11 targets the cystine-rich domain (CRD) of TROP2, which is essential for the tetrameric assembly of TROP2.
K5-70 is another CRD-targeted monoclonal antibody in development. Its key epitope is located on the V43-D65 peptide within the TROP2-CRD. This binding region overlaps with the tetramerization interface of TROP2. K5-70 has the potential to disrupt TROP2 clusters on the tumor cell surface and subsequently interfere with oncogenic signaling pathways. This suggests that CRD-targeted monoclonal antibodies have significant potential in TROP2-positive tumors.
Most reported anti-TROP2 mAbs primarily target the CRD and CPD domains, with fewer targeting the TY domain. Structurally, the TY region is widely involved in TROP2 oligomer assembly and includes conserved sites critical for tumor-specific proteolytic cleavage. Therefore, it is reasonable to speculate that targeting TY may interfere with TROP2-mediated intracellular and intercellular communication, potentially hindering the progression of TROP2-positive tumors.
Bispecific antibodies
Because drug resistance and tumor heterogeneity can limit the clinical outcomes of targeted therapies against a single antigen, a variety of emerging multispecific drugs related to TROP2 (such as bispecific antibodies, trispecific antibodies, and bispecific ADCs) have been evaluated in preclinical studies.

An early reported anti-TROP2 bispecific antibody was a bispecific T cell engager (BiTE), a TROP2-CD3 bispecific antibody that binds to CD3 on T cells and the TROP2 antigen on tumor cells. Activated T cells release cytotoxic cytokines, leading to the lysis of TROP2-positive tumor cells.
F7AK3 consists of an anti-CD3 scFv fused to the Fc region of human anti-TROP2 IgG4. In vitro analyses demonstrated that F7AK3 triggered T cell-mediated cytotoxicity against several TROP2-expressing tumor cell types, with activation levels dependent on TROP2 antigen expression. In a TNBC xenograft model, F7AK3 was shown to inhibit tumor growth through multiple MOAs.
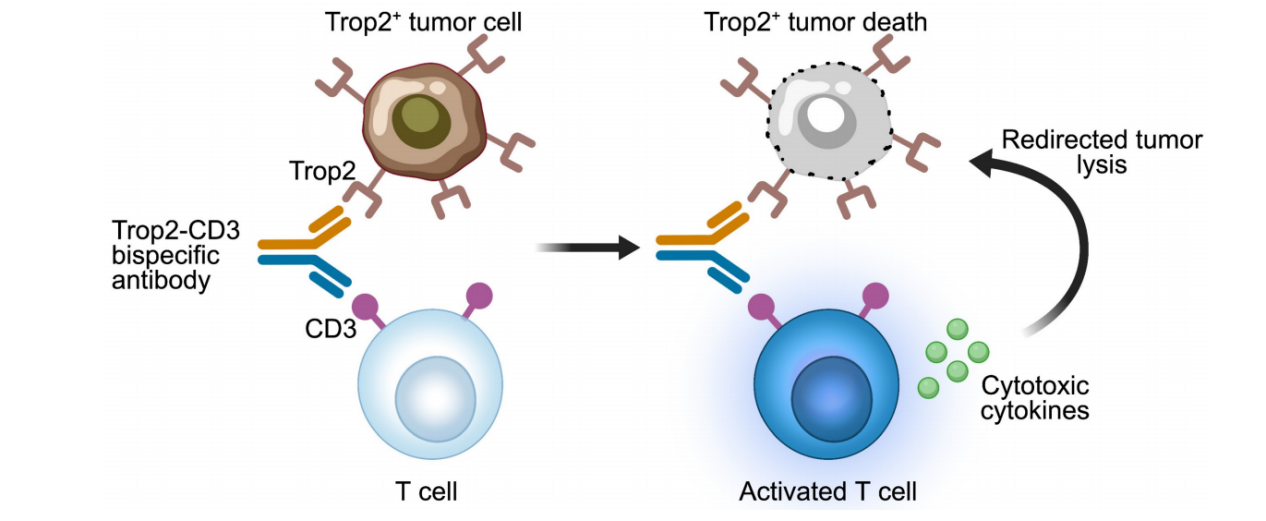
CAR-T/CAR-NK therapy
Currently, many TROP2-specific CAR-T/CAR-NK have shown promising efficacy in the preclinical or early clinical stages.
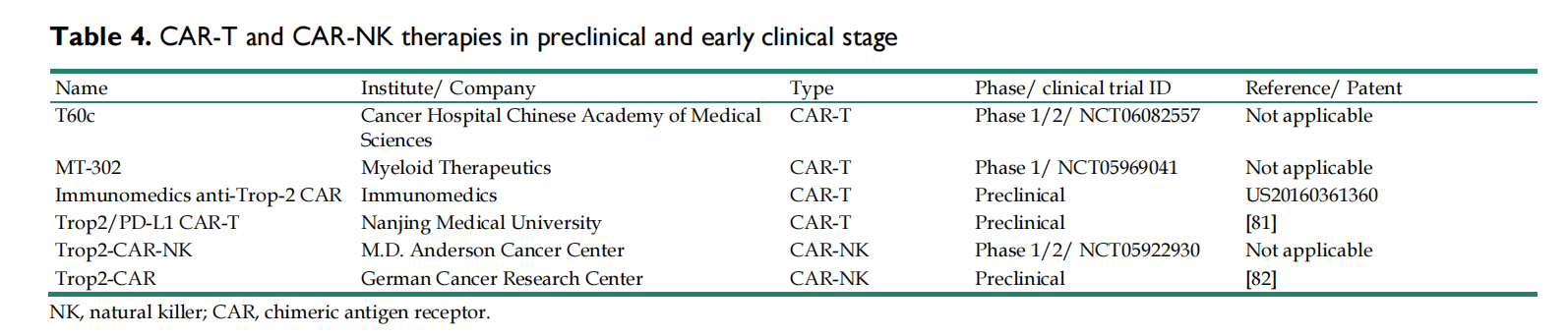
Vaccine
The goal of cancer vaccines is to activate the patient's immune system, enabling it to detect and eliminate tumor cells by inducing B cells and stimulating CD8+/CD4+ T cell responses against tumor-specific antigens. Several TROP2-specific cancer vaccines are currently in preclinical development using different platforms. The TROP2-targeted nanogel vaccine (NIGel-Vax) is a novel cancer immunotherapy.

Summary
TROP2 is overexpressed in cancer cells and has become a promising target for cancer treatment. Advances in ADC-based anti-TROP2 therapies have significantly changed the treatment landscape for patients with TROP2-positive solid tumors. Due to the heterogeneity and individual specificity of tumors, relying on a single drug or therapy is often sufficient to achieve better outcomes. Preliminary data from preclinical and early clinical studies suggest that TROP2-targeted ADCs, when combined with chemotherapeutic agents, small molecule inhibitors, immunotherapy, or anti-angiogenic therapy, can produce synergistic anti-tumor effects in drug-resistant tumors. The additive toxicity of drug-drug interactions may increase the risk of treatment failure. Therefore, finding the optimal combination therapy with good responses and manageable toxicity remains a major challenge.

(Data source Liu X, et al. Theranostics. 2024)
