Background
Escherichia coli is one of the most studied microorganisms in microbiology. Due to its rapid growth, ease of genetic manipulation, and relatively high protein yields, it is widely used in recombinant protein production. Despite these advantages, its inability to efficiently secrete proteins naturally remains a drawback, resulting in protein accumulation in the cytoplasm as inclusion bodies, leading to low overall protein yields. Numerous approaches have been devised to mitigate this weakness and enhance extracellular secretion to increase protein yields.
On March 3, 2025, researchers published an article titled "Advancements in Escherichia coli secretion systems for enhanced recombinant protein production" in World J Microbiol Biotechnol. The article reviews natural and engineered secretion systems in Escherichia coli, highlighting their potential for enhancing the secretion of non-glycosylated proteins. Natural one-step (e.g., type I and type III secretion systems) and two-step systems (e.g., the Sec and Tat pathways) are detailed alongside recent advances in genetic engineering, mutagenesis, and synthetic biology approaches aimed at improving protein yield, folding, and secretion efficiency. Emerging technologies, such as the ESETEC® and BacSec® platforms, offer scalable and cost-effective solutions for higher protein yields. Major challenges, including limited cell capacity and protein aggregation, are addressed through innovative strategies such as cell wall modification, chaperone coexpression, and culture medium optimization. This article also reviews the adaptability of E. coli to industrial applications and the prospects for recombinant protein technology.

Escherichia coli membrane structure
Escherichia coli is a Gram-negative bacterium with a three-layered cell membrane: the inner membrane (IM), the outer membrane (OM), and the periplasmic space.
The periplasmic space: provides a highly oxidative environment that facilitates protein folding, disulfide bond formation, and chaperone-assisted stabilization, all of which are crucial for efficient secretion. Accumulation in the periplasm can sometimes lead to protein aggregation, necessitating further optimization of the secretion pathway.
Outer membrane: Outer membrane proteins (OMPs) typically have a β-barrel structure and are primarily involved in enzymatic reactions and substance transport. The outer membrane's bilayer is composed of phospholipids on the inner side and lipopolysaccharide (LPS) on the outer side. LPS, through its component endotoxin, can be released upon bacterial lysis, triggering an immune response in the host and contributing to bacterial defense.
Cell envelope: Serves as a point of attachment for appendages such as flagella and hairs, aiding bacterial cell movement, communication, and attachment. Envelope proteins participate in both the one-step and two-step secretion pathways.

The natural secretion system of Escherichia coli
One-step secretion system
Normally, E. coli uses the T1SS (type I secretion system) in conjunction with the flagellar T3SS (type III secretion system) for protein translocation in its native state.
Type I secretion systems (TISSs): Composed of an inner membrane ATP-binding protein (ABC), an outer membrane porin (OMP), and a membrane fusion protein (MFP), they form a channel between the cytoplasm and the extracellular space, allowing proteins to completely bypass the periplasm. Using a one-step secretion system for recombinant protein production has several disadvantages. The selected target protein requires a secretion vector (e.g., protein + HlyA); secretion systems rely on the co-expression of HlyB/HlyD for secretion.
Type III secretion system (T3SS): A highly specialized secretory pathway that enables the intracellular delivery of proteins.

Two-step secretion system
The two-step secretion system secretes proteins through the inner membrane and then through the outer membrane. The general secretory (Sec) pathway and the twin-arginine translocase (Tat) pathway are used to transport unfolded and folded proteins from the inner membrane to the periplasmic space, respectively. Proteins are then transferred from the outer membrane to the external environment via the type 2 secretion system (T2SS) and type 5 secretion system (T5SS). The two-step secretion pathway can enhance the secretion of a variety of proteins, and modifications to the outer membrane can promote the release of periplasmic proteins into the extracellular space.
Protein transport across the inner membrane
The Sec pathway: typically transports unfolded proteins. In the Sec pathway, target proteins are coupled to SecA/SecB or signal recognition protein (SRP). With the help of a signal peptide, many proteins enter the Sec translocase present on the IM and are transported to the periplasm . The Sec translocase typically includes SecYEG and SecA proteins, which are involved in post-translational protein secretion. SecA hydrolyzes ATP to provide energy, SecYEG forms a transmembrane channel, and SecB (a molecular chaperone that stabilizes precursor proteins prior to translocation) stabilizes the unfolded protein.
Tat pathway: Transport folded proteins, along with their cofactors, are translocated across the IM via another pathway called the twin-arginine translocase pathway, or Tat pathway. The Tat pathway utilizes TatBC and the Tat(A) complex, which recognize and bind to a specific signal. This leads to the recruitment of various TatA monomers, forming a transmembrane channel for protein translocation. Tat transport is less efficient than Sec transport.

Optimization strategies to improve secretion efficiency
Optimization of signal peptide sequence
Optimizing signal peptides can improve transport efficiency, stability, and specificity to enhance secretion of recombinant proteins. Common strategies include the following:
1)rational design and mutagenesis to modify hydrophobicity and cleavage sites for better secretion;
2)Fusion with carrier proteins such as MBP or OsmY to stabilize the nascent polypeptide and prevent degradation;
3)Through directed evolution screening, efficient signal peptides were identified from genetic libraries;
4)Synthetic signal peptides can be designed using computational tools such as SignalP and TatP;
5)Strain engineering methods, such as co-expression of chaperone proteins or modification of membrane permeability, can further improve secretion efficiency.
Genetic engineering of strains
CRISPR-Cas9 technology can be used to modify the bacterial cell wall to increase membrane permeability and enhance secretion. For example, precise gene knockout or knock-in can be used to regulate the expression of secretion-related genes, such as those encoding chaperone proteins, signal peptides, or outer membrane transporters. Strain optimization can also be achieved by reducing protease activity, minimizing protein degradation, and alleviating cellular stress to improve overall secretion efficiency.
Media optimization and chemical methods
Adjusting the culture medium composition, such as adding glucose and glycerol as carbon sources to provide energy, also plays a significant role in media pH and osmotic pressure. Adding the chemical additive IPTG can promote protein induction, while adding the surfactant Tween-20 can reduce aggregation of hydrophobic proteins. Adding dimethyl sulfoxide (DMSO), glycerol, or betaine can stabilize folding intermediates and reduce misfolding or aggregation. Adding cysteine, glutathione, or dithiothreitol (DTT) can promote disulfide bond formation.
Emerging Technology Platforms
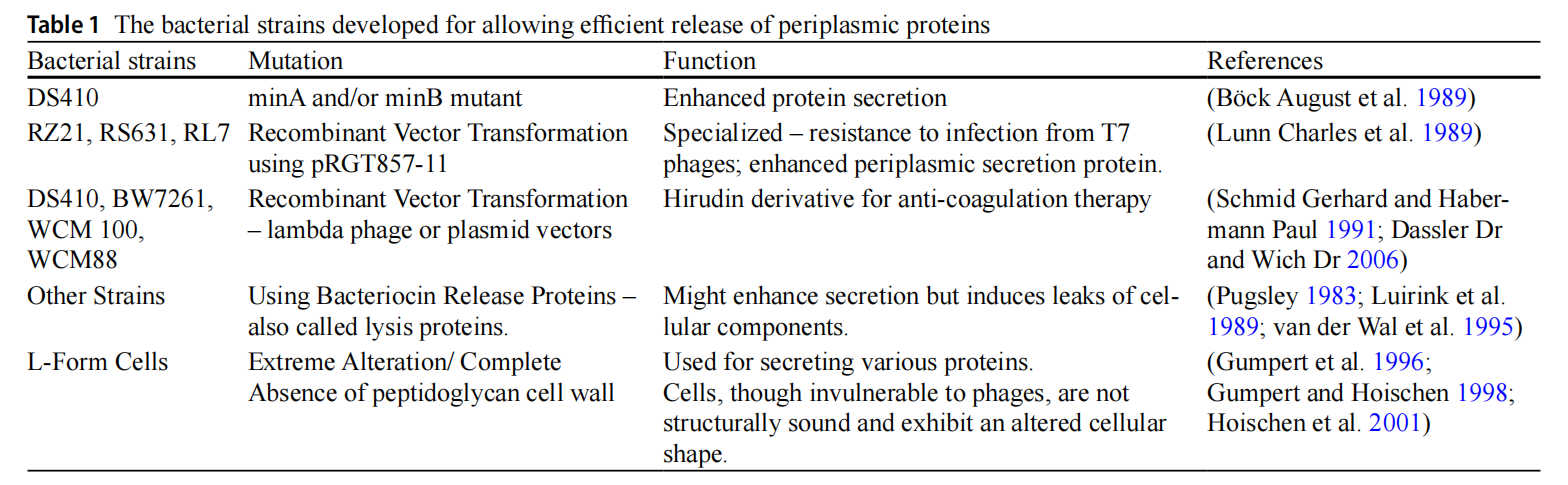
ESETEC® system is an E. coli protein secretion system developed by Wacker and is based on the Escherichia coli K-12.
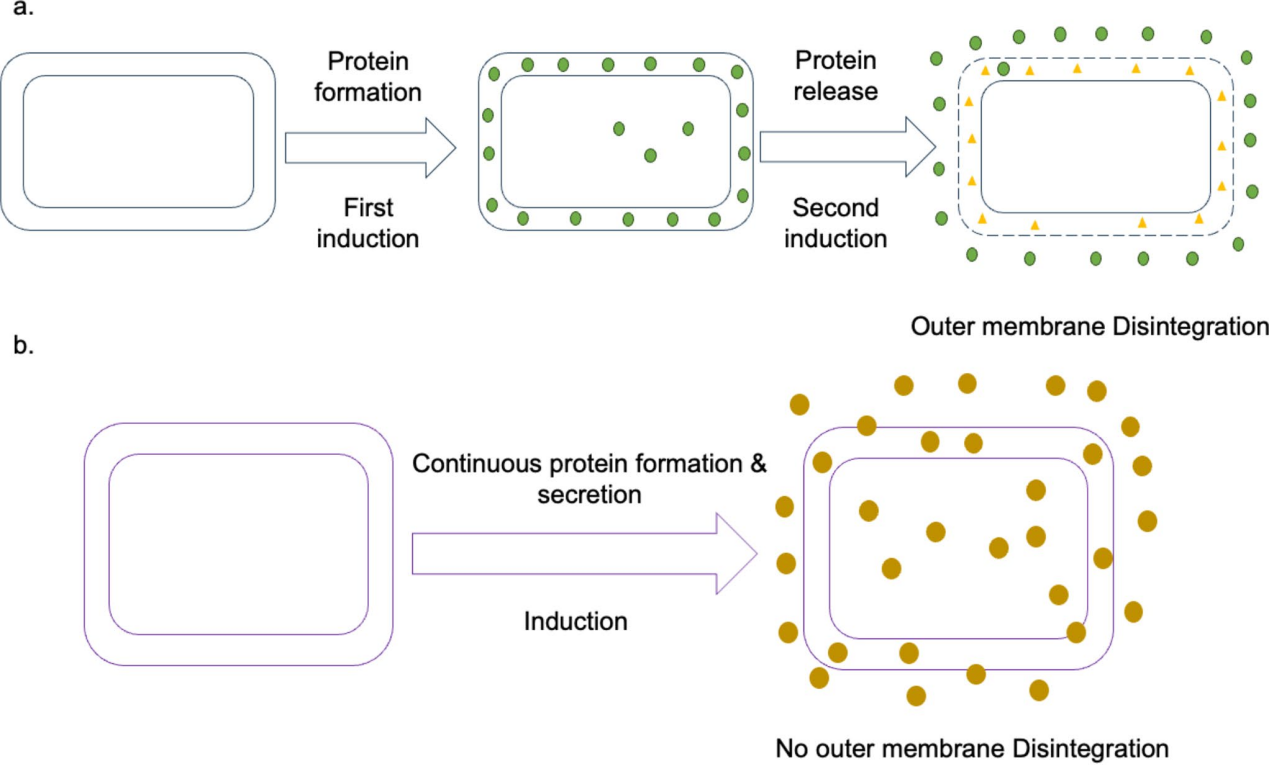
The minA and minB genes in the outer membrane space are knocked out, and the outer membrane and signal peptide are optimized to enable secretion of the recombinant protein after expression. This system can produce non-glycosylated proteins of prokaryotic, eukaryotic, and mammalian origin, ranging in size from 5kD to 150kD.
BacSec ® is a new technology developed by Oncosimis Biotech that integrates upstream and downstream processes, employing a continuous bioprocessing model and perfusion technology to increase protein yield and reduce production costs. This platform enables protein secretion without disrupting the outer membrane, reducing host cell protein contamination.
Summary and Outlook
Advances in genetic engineering, synthetic biology, and process optimization technologies are expected to further improve protein secretion efficiency in E. coli. For example, the development of engineered chaperone systems, the design of hybrid signal peptides, and the adoption of continuous production technologies will drive advancements in recombinant protein production. E. coli holds broad application prospects in recombinant protein production. By optimizing secretion systems and production processes, more efficient and economical protein production can be achieved to meet the needs of both industrial and medical fields.
