Macrophage migration inhibitory factor (MIF) has been discovered as an inhibitor of T lymphocyte induction and acts as a pleiotropic proinflammatory cytokine. It is involved in various functions, including leukocyte recruitment, inflammation, immune response, cell proliferation, tumorigenesis, and counter-regulation of glucocorticoids (GCs).
MIF expression distribution
MIF is mainly expressed in T cells, monocytes, macrophages, dendritic cells, B cells, neutrophils, eosinophils, mast cells and basophils in the blood. In addition, it is also expressed in glandular epithelial cells, squamous epithelial cells, specialized epithelial cells and endocrine cells.

(Data source: Uniprot)
Structure of MIF
MIF is a secreted protein composed of 115 amino acids. It is a highly conserved, non-glycosylated protein with a molecular weight of 12.5 kDa. MIF is a homotrimer, and another member of the MIF superfamily, D-DT/MIF2, is the only homolog with a similar functional spectrum to MIF. MIF is a ligand for CD74, CXCR2, CXCR4, and CXCR7.

(Data source: Kang I, et al. Nat Rev Rheumatol. 2019)
MIF signaling pathway and regulation:
MIF exerts its biological activity in an autocrine or paracrine manner, primarily by interacting with the cell surface receptor CD74 and subsequently recruiting CD44 to form a receptor complex. Furthermore, MIF can bind to chemotactic receptors, including CXCR2, CXCR4, and CXCR7. Following receptor binding, MIF induces activation of downstream signaling pathways, including mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT), and NF-κB. These signaling pathways participate in neuroinflammation by inducing the production of proinflammatory cytokines such as IL-6 and TNF-α, regulating neuronal survival and neuroplasticity. Furthermore, MIF can directly enter the cytoplasm through endocytosis, where it binds to JUN activation domain-binding protein 1 (JAB1), regulating AP-1 activity and cell proliferation. MIF can further translocate to the nucleus by binding to apoptosis-inducing factor (AIF), leading to DNA fragmentation and cell death.

(Data source: Zhang Y, et al. Fundam Res. 2023)
The role of MIF in tumors
The functions of MIF in eliciting pro-tumor or anti-tumor immune responses are diverse and often context-dependent. Generally, during early tumorigenesis and growth, MIF supports a pro-inflammatory immune phenotype controlled by early infiltrating or resident macrophages and inflammatory IL-17-producing T lymphocytes, which together increase inflammation and may lead to cellular and tissue damage. As tumors progress to more advanced, larger, and more immunosuppressive disease, the MIF phenotype begins to resemble more wound-lytic activity, in which case MIF—both tumor cell-derived and immune effector cell-derived—initiates pro-tumor immune evasion and neovascularization processes in a variety of immune cell types.
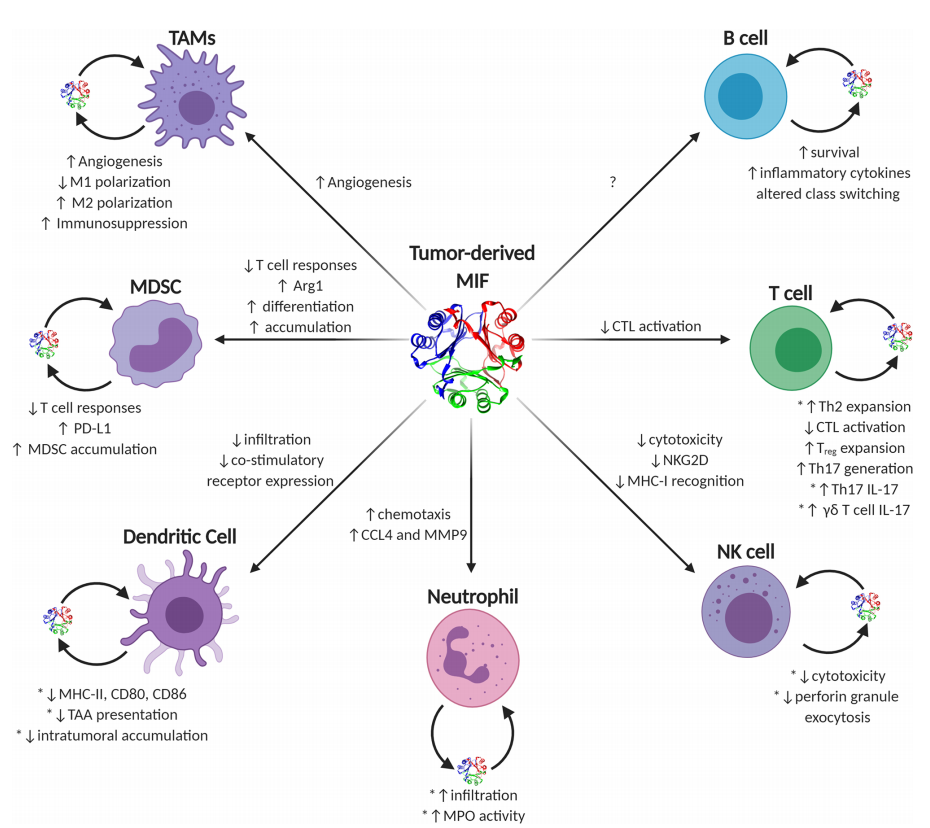
(Data source: Noe JT, Mitchell RA. Front Immunol. 2020)
Targeted therapy for MIF
MIF plays a crucial role in the pathogenesis of inflammatory and autoimmune diseases, and has become an attractive new therapeutic target. The neuroendocrine axis is a crucial regulatory system guiding inflammatory responses, and MIF is the primary protein secreted by anterior pituitary cells upon stimulation. This has drawn researchers' attention to the evaluation of MIF function and MIF-targeted therapies. Currently, MIF-based therapeutic strategies are at the forefront of oncology research. MIF-targeted therapies primarily utilize small molecule inhibitors, although a number of antibodies targeting MIF are currently in clinical development.
Imalumab is a monoclonal antibody targeting MIF used for the treatment of colorectal cancer and ascites. A phase 1 study (NCT01765790) evaluated the safety, pharmacokinetics, tolerability, and antitumor activity of imalumab in solid tumors. The maximum tolerated dose of imalumab was 37.5 mg/kg (intravenously) every 2 weeks, and the dose-limiting toxicity was allergic alveolitis in patients with solid tumors. Imalumab was also evaluated in patients with ovarian cancer and malignant ascites (NCT02540356) and in combination with 5-FU/leucovorin or panitumumab in patients with metastatic colorectal cancer (mCRC) (NCT02448810). However, both studies were terminated prematurely due to design deficiencies and overall benefit-risk assessment.
