Background
The dendritic cell inhibitory receptor (DCIR) is a C-type lectin receptor selectively expressed on myeloid cells, including monocytes, macrophages, dendritic cells, and neutrophils. Its role in immune regulation has been demonstrated in mouse models and human genome-wide association studies (GWAS), showing that DCIR deficiency is associated with increased susceptibility to autoimmune diseases such as rheumatoid arthritis, lupus, and Sjögren's syndrome. However, little is known about the mechanisms by which DCIR activation suppresses inflammation.
On May 23, 2024, researchers published an article in JCI Insight titled "Agonistic anti-DCIR antibody inhibits ITAM-mediated inflammatory signaling and promotes immune resolution." American researchers developed anti-DCIR agonistic antibodies to promote phosphorylation of the DCIR immunoreceptor tyrosine inhibitory motif (ITIM) and recruitment of SH2 containing tyrosine phosphatase-2 (SHP2) to reduce inflammation. The researchers also explored the use of antibodies to deplete DCIR+ cells to resolve inflammation. Using a human DCIR knock-in mouse model, the researchers validated the anti-inflammatory effects of anti-DCIR agonistic antibodies on experimental peritonitis and colitis. These findings provide key evidence for the development of transformative therapies targeting DCIR for inflammatory diseases.

Overview of Dendritic Cell Inhibitory Receptor (DCIR)
Immunomodulatory receptors play a crucial role in immune homeostasis by balancing activation and inhibition of immune responses. Inhibitory receptors typically transduce inhibitory signals through immunoreceptor tyrosine-based inhibitory motifs (ITAMs) located within the cell to counteract the function of neighboring immunoreceptor tyrosine-based activation motifs (ITAMs) receptors. Dendritic cell inhibitory receptors (DCIR) contain ITIM motifs with extracellular lectin-like domains and cytoplasmic domains, providing anti-inflammatory signals by recruiting phosphatases SHP-1 and SHP-2. DCIR-/- mice are highly susceptible to disease in collagen-induced arthritis (CIA) and experimental autoimmune encephalomyelitis (EAE) models. In addition, DCIR polymorphisms are associated with patient susceptibility to autoimmune diseases such as rheumatoid arthritis (RA). Systemic lupus erythematosus (SLE), and primary Sjögren's syndrome. Therefore, insufficient DCIR function may lead to autoimmunity. Studies have shown that dendritic cells and DCIR receptor on macrophages inhibits TLR-dependent inflammatory cytokine production, such as IL-1β, IL-6, TNFα, IL-12, and IFNα. Sialyl IgG ligation to DCIR generates tolerogenic DCs and Tregs. A recent study demonstrated that binding of an N-glycan (NA2) to DCIR on DCs ameliorates symptoms of CIA and EAE.

(Data source: Kaifu T, et al. J Exp Med. 2021)
DCIR is induced in diseased cells
Comparing DCIR mRNA expression in unperturbed blood immune cells using the BLUEPRINT dataset revealed that DCIR expression is enriched not only in immature and mature conventional DCs (cDCs), but also in neutrophils, classical monocytes, and macrophages. However, DCIR expression is low in B cells and T cells. Like other immune checkpoint receptors, elevated DCIR following stimulation may serve as a feedback mechanism to prevent excessive inflammation in activated immune cells. DCIR protein is further induced by LPS stimulation in inflammatory states, but DCIR is also rarely found on B cells and T cells. scRNAseq analysis revealed that in lesional HS samples, DCIR is enriched in tissue-infiltrating neutrophils, monocytes, macrophages, and tissue-resident myeloid cells, including cDC1/2 and Langerhans cells.

An RNA-seq analysis revealed elevated DCIR levels in autoimmune disease (CD), ulcerative colitis (UC), and HS compared with healthy controls. In addition to HS, scRNAseq data from mucosal tissues of CD patients resistant to anti-TNFα therapy revealed that DCIR is selectively expressed in classical and intermediate monocytes and DCs within lesions. Large-scale RNAseq data from mucosal tissues further support the association between increased DCIR expression and resistance to anti-inflammatory therapy.

Consistent with the RNAseq results, immunohistological staining also confirmed increased accumulation of DCIR+ cells in the epidermis and dermis of HS skin biopsies, CD mucosal tissue, and cutaneous lupus lesions. The high expression of DCIR in disease provides a theoretical basis for the development of DCIR-based therapeutics to inhibit the migration and activation of inflammatory myeloid cells.

Identification of human anti-DCIR monoclonal antibodies
To investigate the potential of DCIR as a therapeutic target for inflammatory diseases, researchers generated a DCIR monoclonal antibody. During antibody production, culture supernatants from hybridoma clones were isolated to identify secreted antibodies that bound to HEK293 cells overexpressing huDCIR and monocyte-derived dendritic cells (MoDCs). The heavy and light chain variable regions (VH and VL) from hybridomas secreting strong DCIR binders were cloned into a human IgG1 backbone and expressed as chimeric monoclonal antibodies. Cross-reactivity with cynomolgus (cyno) DCIR and huDCIR was confirmed, but not with mouse DCIR1 (muDCIR1). Finally, after removal of rat-derived sequences and codon optimization according to AbbVie's high-throughput antibody humanization design software, a fully humanized antibody was generated. It was found to be specific for human and cynoDCIR, but not for other selected human C-type lectin receptors, pattern recognition receptors, and muDCIR1 expressed on the mouse DC cell line JAWSII.

Anti-DCIR mAb induces tyrosine phosphorylation
The interaction between DCIR tyrosine phosphorylation and SHP2 was identified by co-immunoprecipitation. Anti-DCIR monoclonal antibodies were screened in huDCIR-overexpressing HEK293 cells; clones 9D9, 3B4, and 3A4 induced robust agonism. Researchers generated a HuDCIR agonistic signaling reporter cell line, replacing the DCIRITIM motif. Antibody-mediated agonism was quantified by measuring luciferase activity through activation of ITAMNF-κB signaling. Mannose and NA2 -glycans, ligands for DCIR tolerance induction, were identified. Mannose and NA2 -glycans bound to BSA and immobilized on a plate induced agonism, reaching saturation at 50 µg/ml using a DCIR agonistic signaling reporter system. Pretreatment of mannose-BSA or NA2-glycan-BSA (50 µg/ml) followed by the addition of an agonistic anti-DCIR antibody (9D9) did not impair 9D9-induced signaling. These results suggest that anti-DCIR antibodies further amplify DCIR's immune tolerance signaling, even when occupied by the natural ligand.

Agonistic anti-DCIR antibodies exert immunosuppressive functions
To ensure that the agonistic effects were not due to differential antibody binding, the researchers confirmed comparable antibody binding to human monocytes. Consistent with antibody screening results in HEK293 cells overexpressing DCIR, the agonistic anti-DCIR mAbs 9D9 and 3A4 triggered the interaction between DCIR and SHP2 in human monocytes. Stimulation of monocytes with immune complexes against human serum albumin (IC:HSA) in the presence of an Fc-matched isotype control or the non-agonistic anti-DCIR mAb 3F7 increased the interaction between SYK and SHP2. Treatment of immune complex-stimulated monocytes with the agonistic anti-DCIR mAbs 9D9 and 3A4 significantly reduced the SHP2-SYK interaction to levels similar to those in unstimulated cells, while promoting the interaction between SHP2 and DCIR. These data suggest that the DCIR-ITIM signaling pathway may overcome ITAM activation and compete for SHP2 recruitment. Indeed, the agonistic anti-DCIR clones 9D9 and 3A4 also impaired the interaction between SYK and the Fc receptor γ chain. Both agonistic antibodies (9D9 and 3A4) reduced OCR compared with isotype antibodies and non-/weakly agonistic antibodies (3F7 and 5E11).

Agonistic mAbs 9D9 and 3A4 inhibited DECTIN1 activation upon zymosan-d (ZymD) stimulation, as indicated by decreased TNFα and IL6 secretion from monocytes and PBMCs, whereas the non-agonistic mAb 3F7 had no effect.

Infiltrating DICR-positive cells can be seen in huDCIR-ki mice
The researchers designed a huDCIR-ki mouse to evaluate the potential of targeting huDCIR as a treatment for inflammatory diseases. During acute peritonitis and colitis, infiltrating DCIR-positive cells were seen in huDCIR-KI mice. In the ZymD-induced peritonitis model, the researchers observed a large number of huDCIR-positive cells in the peritoneal fluid of huDCIR-KI mice. In the DSS-induced colitis model, the researchers also found a large number of huDCIR-positive cells infiltrating the colonic crypts. These results indicate that DCIR-positive cells are significantly increased in these inflammatory disease models, indicating that DCIR may play an important role in the pathogenesis of these diseases.

Agonist anti-DCIR monoclonal antibody improves experimental acute peritonitis
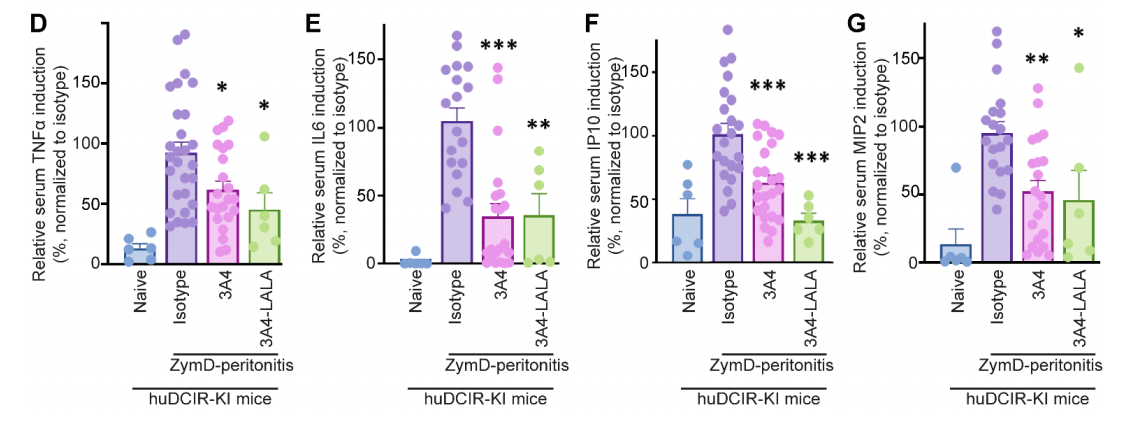
The researchers evaluated the efficacy of agonistic and non-agonistic mAbs in a ZymD - induced peritonitis model. They found that prophylactic treatment with agonistic clones 3A4 and 9D9 significantly reduced the severity of peritonitis in huDCIR-KI mice compared with Fc-matched isotype controls, as evidenced by reduced accumulation of leukocytes and neutrophils in peritoneal lavage fluid. No significant effect of agonistic clone 3A4 treatment was observed in WT mice.

To eliminate the influence of the antibody Fc portion on the evaluation of agonistic effects, the researchers compared WT 3A4 with 3A4 variants with impaired FcR binding ability caused by LALA (L234A and L235A) mutations and found that strong agonism had a more obvious protective effect on DCIR than FcR binding.
Anti-DCIR antibodies promote neutrophil clearance via ADCP and ADCC
LPS-induced neutrophils were labeled with CellTrackerGreen dye and co-cultured with human monocyte-derived macrophages in the presence of a panel of anti-DCIR antibodies or Fc-matched isotype controls. It was found that the agonistic clones 3A4 and 9D9, as well as the weakly agonistic clone 5E11 and, to a lesser extent, the non-agonistic clone 3F7, all exhibited enhanced ADCP effects. Using a commercial ADCC reporter system, it was found that 3A4, 9D9, and 5E11 could also promote neutrophil clearance through Fc-mediated ADCC, and 3A4 The LALA mutant failed to trigger ADCC-related signaling.

No significant endocytosis was observed with anti-DCIR mAbs. These data suggest that our anti-DCIR mAbs may provide a method to overcome DCIR endocytosis and promote antibody-mediated clearance of inflammatory DCIR+ cells. To further evaluate antibody-mediated neutrophil clearance in vivo, the researchers compared wild-type and LALA-mutated 5E11 clones in a ZymD-induced mouse peritonitis model. Compared with LALA, wild-type 5E11 significantly reduced the accumulation of leukocytes and neutrophils in the peritoneal cavity.

To maximize the ADCP and ADCC effects mediated by the mouse Fc receptors CD16 and CD32, the researchers also prepared a mouse IgG2b version of the 5E11 antibody (5E11-mIgG2b). In a ZymD-induced peritonitis model, 5E11-mIgG2b significantly reduced the accumulation of leukocytes and neutrophils in the peritoneal cavity and inhibited the production of the inflammatory cytokines IL-6 and TNF-α, demonstrating comparable efficacy to anti-Gr-1 antibodies.

Agonist anti-DCIR mAb alleviates experimental colitis
Researchers treated mice with an agonist DCIR monoclonal antibody (9D9), a non-agonist monoclonal antibody (3F7), and an isotype control before and three days after DSS exposure. They found that the agonist anti-DCIR monoclonal antibody 9D9 significantly attenuated DSS-induced colonic inflammation, as evidenced by reduced weight loss. In the DSS-induced colitis model, the extent of neutrophil activation and crypt abscesses correlated with disease severity. Confocal laser endoscopy (CLE) revealed that 9D9 reduced neutrophil activation and damage to densely packed ducts in the colon. Histological analysis also revealed that 9D9 treatment reduced ulceration and inflammatory cell infiltration in the colonic mucosa, as well as proliferation of mucosal glands and submucosa. 9D9 also significantly reduced secretion of the inflammatory cytokine MIP-2, suggesting that it reduces neutrophil chemotaxis and activation. Overall, administration of an agonist anti-DCIR monoclonal antibody significantly ameliorated DSS-induced colitis.

Summary
DCIR agonistic antibodies recruit SHP2 to its intracellular ITIM domain, thereby inhibiting activation of the immunostimulatory SHP2-SYK pathway, typically initiated by other proinflammatory pattern recognition receptors. Anti-DCIR antibodies may promote resolution of inflammation through Fc-mediated neutrophil clearance. These findings reveal a mechanism of immunosuppression by anti-DCIR monoclonal antibodies, both through agonism and cell depletion, laying a solid foundation for transformative treatments targeting DCIR for inflammatory diseases. Compared with targeting a single receptor, crosslinking multiple immunosuppressive receptors using multivalent antibodies can produce a more potent inhibitory effect. Future studies could explore the use of bispecific agonistic antibody design, clustering DCIR with another immunosuppressive receptor, such as PD-1, or utilizing engineered Fc regions to enhance crosslinking with FcγIIB. These strategies have the potential to significantly improve immunomodulatory function by enhancing co-inhibitory complexes.
