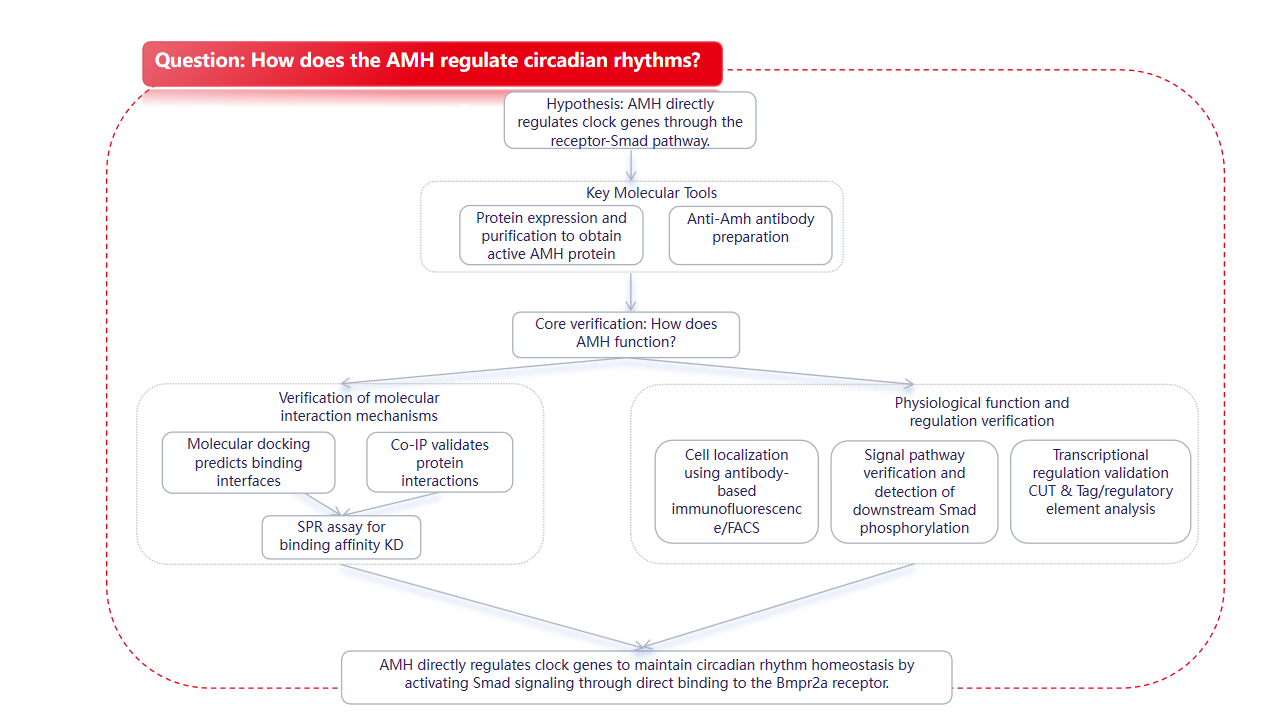
Service Support
Mabnus Biotech provides services for recombinant protein expression, antibody preparation, molecular docking, and SPR detection.

Background
The biological clock coordinates behavior and physiological rhythms in a chronological order. While the core molecules constituting the biological clock are well-defined, the key signaling pathways that lead to or promote homeostasis remain largely unknown. Disruptions to biological rhythms due to mutations in clock genes or abnormal environmental stimuli can lead to endocrine disorders of the female reproductive system, such as polycystic ovary syndrome (PCOS) and premature ovarian failure (POI). Abnormal circulating levels of AMH (anti-Müllerian hormone) may be a contributing factor to the core pathophysiology of various reproductive disorders and have been used as a biomarker for clinical assessment of ovarian reserve and diagnosis of female reproductive diseases such as PCOS, POI, and granulosa cell tumors of the ovary (GCT).

On May 10, 2025, the team led by Mei Jie and Gui Jianfang from Huazhong Agricultural University published a research paper entitled "Anti-Müllerian hormone signalling sustains circadian homeostasis in zebrafish" in Nature Communications. The study found that anti-Müllerian hormone (Amh)-mediated signaling plays a crucial role in maintaining circadian homeostasis in zebrafish. Amh-mediated signaling maintains molecular clock oscillations in the pituitary gland, thereby maintaining circadian homeostasis at the tissue and systemic levels. Amh binds to its receptors Bmpr2a/Bmpr1bb, which then activates Smad1/5/9 through phosphorylation and promotes the expression of circadian rhythm genes. Our findings reveal a key hormone signaling pathway for zebrafish circadian homeostasis, influencing the function of rhythmic organs and circadian rhythm health.
Amh is essential for maintaining the zebrafish's biological clock
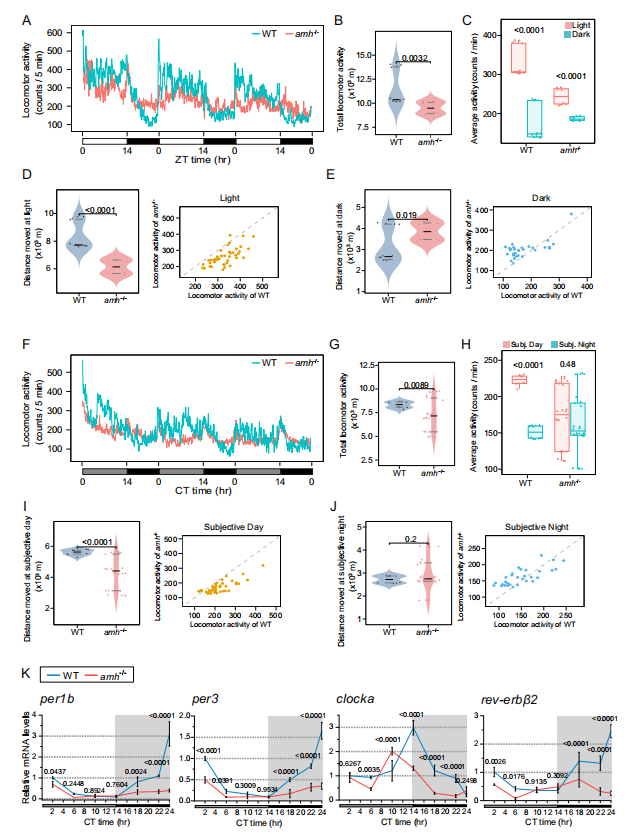
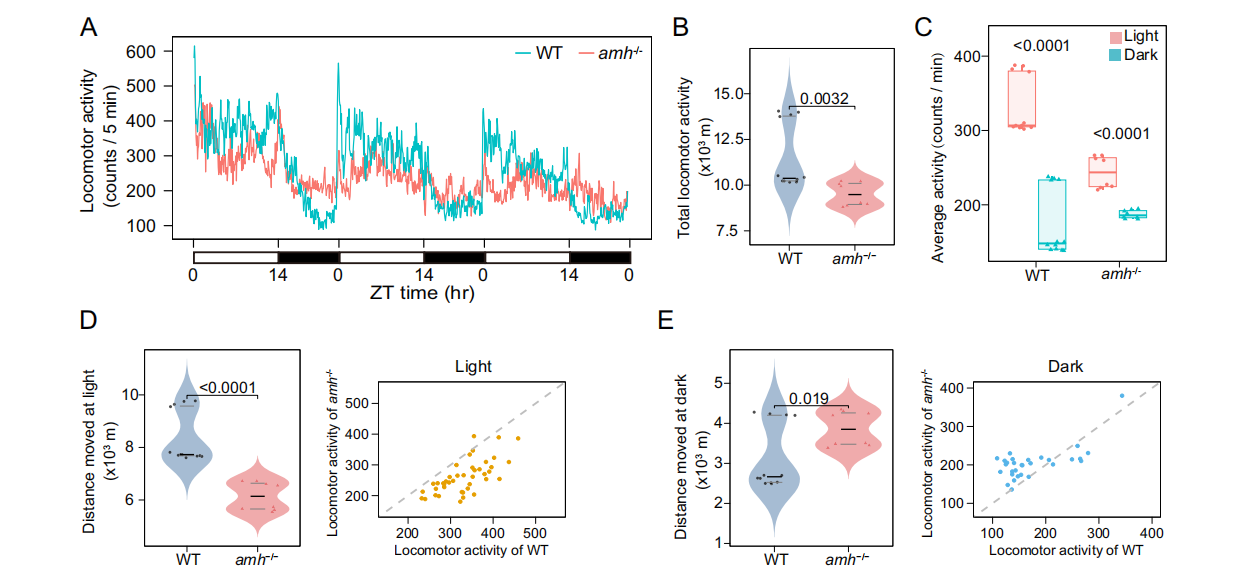
The potential role of Amh in circadian rhythm homeostasis was investigated by monitoring the kinetic activity of wild-type and Amh mutant adult zebrafish under a 14-hour:10-hour light/dark (LD) cycle. Wild-type Amh zebrafish exhibited a strong circadian rhythm of kinetic activity, primarily active during the day and exhibiting sleep-like behavior at night; while the overall activity of the Amh mutant was significantly reduced. This indicates that the rhythmic kinetic activity of the Amh mutant is disrupted, suggesting that zebrafish require Amh to maintain normal circadian behavioral rhythms. Compared to the wild type, the amplitude of the rhythmic expression of clock genes in the pituitary gland of the Amh mutant zebrafish was significantly weakened, indicating that AMH plays an important role in pituitary clock regulation.
Distribution of Amh protein in specific pituitary cell populations and its role in endocrine diurnal homeostasis
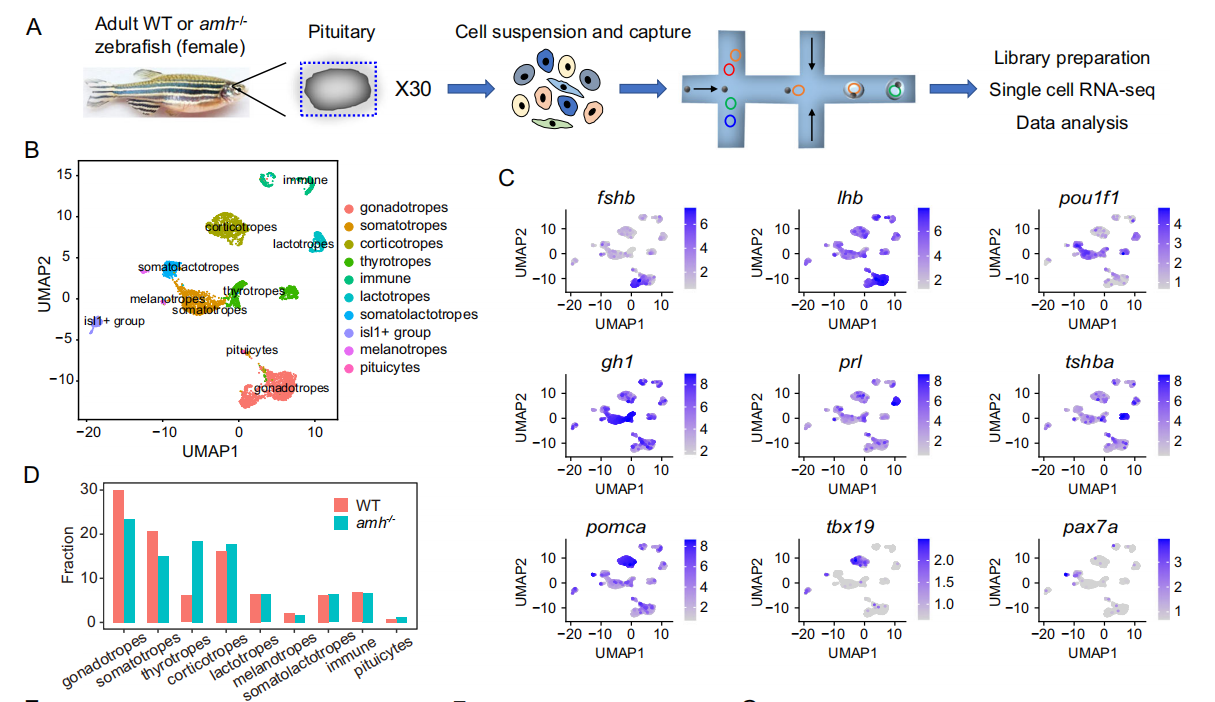
Immunofluorescence staining with Amh-specific antiserum was used to determine the expression of Amh protein in the pituitary gland of adult female zebrafish. Amh-immunoreactive cells were found to be widely distributed in the anterior pituitary gland of WT zebrafish, including the proximal distal pituitary (PPD) and rostral distal pituitary (RPD). After immunofluorescence staining with anti-Amh antibody, Amh-positive pituitary cells were isolated by FACS. All classical endocrine cell marker genes in the PPD and RPD regions of the pituitary gland were expressed in Amh-positive cells. The transcription levels of gh1 (growth hormone cells), lhb, and fshb (gonadotropic cells) were significantly higher than those of other marker genes, such as tshba (thyroid-stimulating cells), pomca (adrenocorticotropic cells and melanocytes), and prl (milk cells), indicating that growth hormone cells are likely the main site of Amh protein expression.
In Amh -deficient pituitary glands, the expression rhythms of these hormone genes (including gh1, lhb, fshb, and tshba) are significantly disrupted. Therefore, Amh is essential for maintaining endocrine circadian rhythm homeostasis.

Amh's lineage-specific regulation of the pituitary circadian rhythm
Single-cell sequencing revealed that many clock genes in the Amh mutant were significantly downregulated, primarily in gonadotropins, growth hormone, thyroid-stimulating hormone, and adrenocorticotropic hormone. Comprehensive scRNA-seq analysis indicated that Amh plays a crucial role in pituitary cell population development and lineage-specific regulation of the biological clock, particularly in Amh-positive pituitary cell populations.

Amh functions in the pituitary gland through the Bmpr2a/Bmpr1bb signaling pathway
Amh (Amh receptor agonist) binds to its specific type II receptor, AMHR2, which in turn heterodimerizes with one of several type I receptors, such as Acvr1 (Alk2), Bmpr1a (Alk3), or Bmpr1b (Alk6), thereby regulating target gene expression. In fish lacking AMHR2, Bmpr2a may be recruited as the Amh receptor, and it is more functionally conserved than Bmpr1b. Bmpr1b (Alk6) is the only type I receptor that interacts with AMHR2 in a ligand-dependent manner. The effects of Amh on the pituitary gland may depend on its receptors Bmpr2a and Bmpr1b b.

Molecular docking analysis and SPR experiments revealed that the C-terminal domain of Amh interacts with the protein kinase domain of Bmpr2a. When full-length Amh was used, binding affinity significantly increased, indicating that the N-terminal domain enhances the interaction between the C-terminal domain of Amh and the protein kinase domain of Bmpr2a.

Function of Bmpr2a in zebrafish
Locomotion experiments were conducted on adult zebrafish with WT and B mpr2a mutations (bmpr2a−/−). The results showed that the locomotor rhythm of the B mpr2a mutant was severely disrupted, and the knockout of Bmpr2a disrupted the biological clock and endocrine homeostasis of zebrafish.
B mpr2a−/− fish was significantly reduced, and core transcription activators and two negative feedback pathway genes, per1b, per3, clocka and rev-erbβ2, were significantly downregulated at multiple time points.
The experiment also found that knockout of B mpr2a also affects the expression patterns of hormone genes in the Amh target pituitary cell line, including hormone-encoding genes such as gh1, lhb, fshb, and tshba, and the expression rhythms of these hormone-encoding genes are severely disrupted.
either Amh or Bmpr2a does not lead to significant morphological abnormalities during embryonic development. Amh regulates the zebrafish circadian rhythm in vivo through its receptor Bmpr2a.

The Amh/Bmpr2a-Smad signaling pathway regulates the transcription of the biological clock
The Amh-Bmpr2a axis is essential for the binding of P-Smad1/5/9 to the promoters of circadian clock genes. Studies have found that the P-Smad1/5/9 binding sites contain typical transcriptional regulatory elements of circadian clock genes, including E/E'-boxes, REV-ERB/ROR binding elements (RRE), cAMP response elements (CRE), and DBP/E4BP4 binding elements. The Amh-Bmpr2a-Smad1/5/9 axis transmits signals by specifically targeting a subset of typical circadian clock gene cis-regulatory elements, thereby regulating the expression of circadian clock genes. Rhythmic Amh signaling maintains circadian rhythms through the Bmpr2a-Smad1/5/9 pathway. Amh-Bmpr2a regulates the expression and oscillation of circadian clock genes through Smad1/5/9-mediated transcriptional regulation, thereby maintaining homeostasis of the internal timing and endocrine systems.

Summarize
Amh (AMH) binds to its receptors (Bmpr2a/Bmpr1bb) and recruits Smad1/5/9 proteins, which are subsequently phosphorylated and translocated to the nucleus. These proteins regulate clock gene expression through multiple time-controlled elements, including E/E'-boxes, RREs, and CREs, providing a molecular mechanism for the regulation of clock gene expression by hormones. The Amh/Bmpr2a-Smad1/5/9 signaling pathway may function as an independent regulator of the circadian rhythm clock, independent of light input pathways. This study also reveals that Amh is an essential endogenous factor for the oscillation of molecular clocks in the pituitary gland and the maintenance of hormonal and behavioral rhythms. These findings suggest that the pituitary clock may be part of a multi-component clock system in the fish brain, opening new avenues for research into the mechanisms of the circadian system in fish.

