Tumor hypoxia remains a significant challenge for the effective treatment of most cancers. Tumor cells in hypoxic environments often exhibit strong resistance to most treatment modalities, and their adaptation to the hypoxic microenvironment endows them with aggressive and invasive behaviors. Carbonic anhydrase 9 (CA9), also known as CAIX or renal cell carcinoma-associated antigen G250, is a dimeric, membrane-bound metabolic enzyme belonging to the carbonic anhydrase (CA) family. It catalyzes the interconversion between carbon dioxide and water, as well as the breakdown of carbonate ions (i.e., bicarbonate and hydrogen ions). CA9 is a hypoxia-inducible protein selectively expressed in hypoxic tumor cells and is a major metabolic effector of tumor hypoxia, regulating intracellular and extracellular pH and acidosis. Targeting CA9 plays a crucial role in cancer treatment.

(Data source: Becker HM. Br J Cancer. 2020)
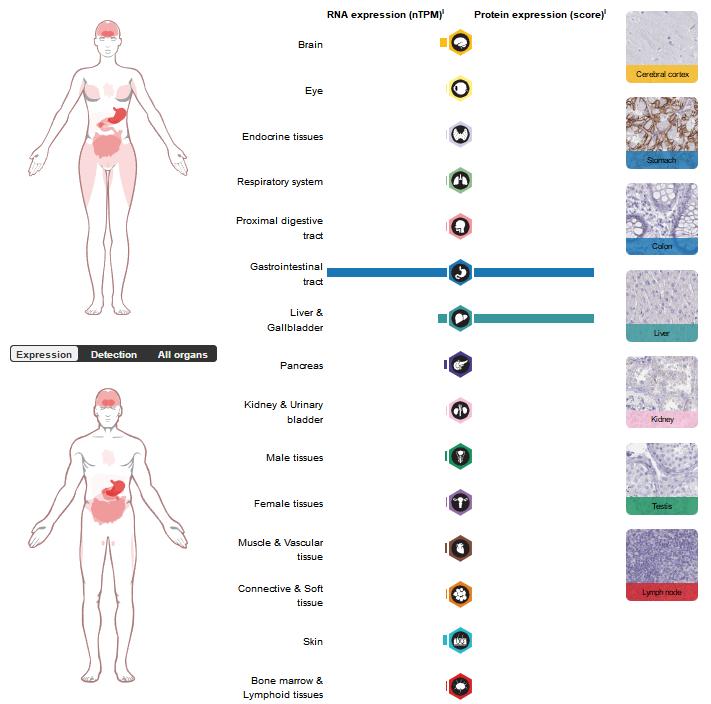
CA9 expression distribution
CA9 expression is tissue-specific, with low expression in normal tissues. It is mainly expressed in the gastrointestinal tract, in spermatogenic cells and gastric gland cells. It is primarily expressed on the surface of tumor cells in hypoxic microenvironments, and is highly expressed in various types of tumor tissues, including breast, kidney, lung, pancreas, colon/rectum, oral cavity (head/neck), cervix, gallbladder, brain, liver, and gastric epithelium.

(Data source: uniprot)
CA9 structure
CA9 is an I CAIX is a transmembrane protein composed of 459 amino acids. It comprises four domains: a signal peptide, an N-terminal proteoglycan-like domain (PG domain), a CA domain, a TM domain, and an IC domain. The PG domain, unique to CAIX within the CA family, is the core region of enzyme activity, containing a crucial zinc ion binding site and efficiently catalyzing the hydration of CO₂ to HCO₃⁻ and H⁺. CAIX typically exists as a dimer , and this dimerization is mediated by disulfide bonds formed between Cys-41 residues located on the CA domain. CAIX is a catalytically active isoenzyme with a histidine residue (His64) at the active site ingress. These residues primarily function in the proton transfer process of highly active CAs.

(Data source: Queen A, et al. Semin Cancer Biol. 2022)
CA9 signal transduction regulation in the tumor microenvironment
Under hypoxic conditions, cancer cells upregulate CAIX expression via HIF-1. CAIX regulates the activity of ion transporters to remove acid or carbon dioxide from the intracellular space. Glycolysis and mitochondrial respiration produce metabolic acids and lactate, which are released into the extracellular space via monocarboxylic acid transporters (MCTs). CAIX hydrates external carbon dioxide and converts it into bicarbonate and hydrogen ions. The intracellular protons consumed by the introduced bicarbonate help raise the intracellular pH, thereby supporting cellular metabolic processes, signaling, and proliferation. In contrast, extracellular protons generated by CAIX-catalyzed hydration (generated by CAIX) remain outside the cell, leading to acidification of the cellular microenvironment, which promotes cancer cell invasion or metastasis. In addition to CAIX, HIF1α also increases the expression of CXCR4 and VEGF. CAIX regulates the activity of CXCR4, JAK, and VEGFR, proteins that activate PI3K and MAPK signaling, thereby supporting metastasis and angiogenesis. The CXCL12-CXR4 axis is a potential chemokine signaling pathway that plays an important role in the metastasis of various cancers and is regulated by hypoxia and CAIX.

(Data source: Queen A, et al. Semin Cancer Biol. 2022)
CA9-targeted therapy
Since CA9 is primarily expressed in cancer cells and expressed at lower levels in normal tissue cells, targeting CA9 is an effective strategy for cancer treatment. Hypoxic tumors exhibit high levels of CAIX and VEGF signaling, which contribute to tumor growth and angiogenesis. Targeted therapy against CAIX reduces VEGF/angiogenesis signaling because tumor cells cannot adapt to the hypoxic environment, leading to reduced angiogenesis and proliferation, and inducing cancer cell death. Currently, the main treatment modalities for CA9 include small molecule inhibitors and antibody immunotherapy.

(Data source: Queen A, et al. Semin Cancer Biol. 2022)
Girentuximab is a monoclonal antibody targeting CA9 for the treatment of urothelial carcinoma, renal cell carcinoma, and renal tumors. It is currently in Phase 3 clinical trials. Although the trial results have not yet met the criteria for clinical approval, it is clear that some patients will benefit from treatment targeting CAIX. These findings have sparked the potential to use anti-CAIX antibodies with radioligands and toxic loads as antibody-drug conjugates (ADCs).
177Lu girentuximab is the first antibody-radioisotope specifically designed for clear cell renal cell carcinoma (RCC). It targets carbonic anhydrase IX-expressing cells, presenting targeted radiation to the cancer while minimizing damage to surrounding healthy cells. As monotherapy for metastatic clear cell renal cell carcinoma, 177Lu girentuximab has safely and effectively stabilized the disease in 57% of patients. At the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, Illinois, Dr. Eric Jonasch presented the ongoing STARLITE-1 study, a phase Ib/II trial of 177Lu girentuximab in combination with cabozantinib and nivolumab in treatment-naïve patients with advanced clear cell renal cell carcinoma (ccRCC). The study evaluates the safety and efficacy in patients with advanced ccRCC.
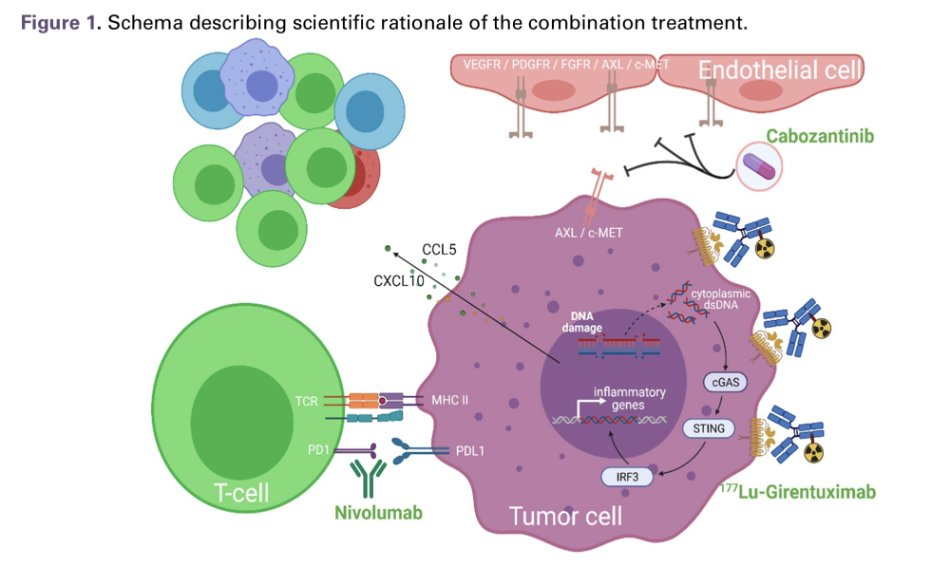
(Data source: ASCO)
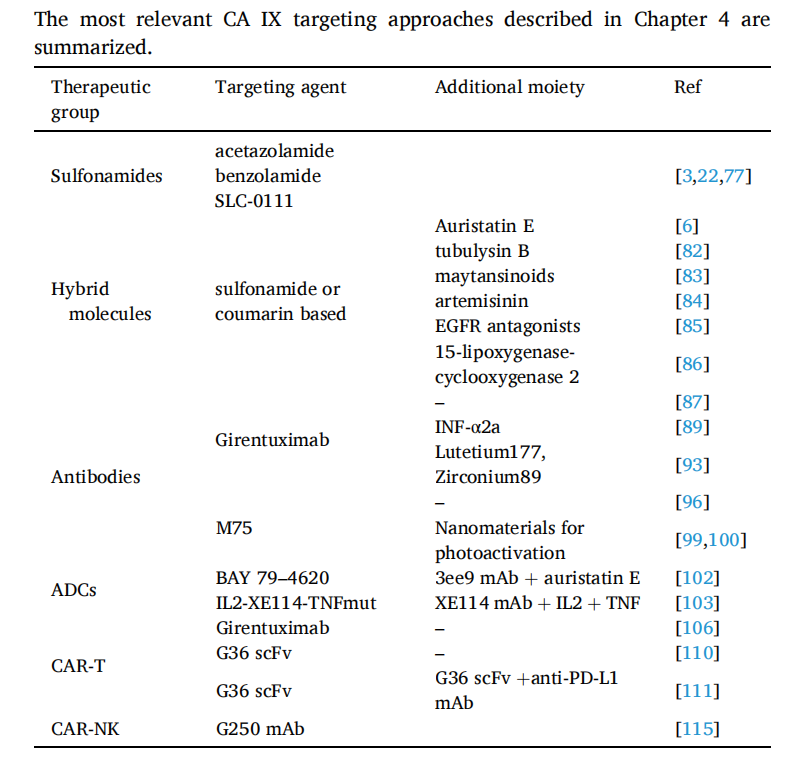
(Data source: Ronca R, et al. Biochim Biophys Acta Rev Cancer. 2024)
