CFP (Properdin) is a positive regulator of the complement alternative pathway (AP). It binds to and stabilizes the C3 and C5 convertase complex, inhibiting CFI-CFH-mediated degradation of the complement C3β chain (C3b). CFP plays a central role in innate immunity, inflammation, and tissue homeostasis.
CFP expression distribution
CFP is mainly expressed in monocytes, dendritic cells, glial cells, neurons, and neutrophils.

(Data source: uniprot)
The structure and receptor of CFP
CFP is a secreted protein composed of 469 amino acids. Its monomer is a glycoprotein of approximately 53 kDa, composed of multiple tandem thioester protein (TSP) domains. In plasma, it exists as a dimeric, trimeric, or tetrameric cyclic multimer (primarily a trimer). This multimerization is crucial for its function. The TSP domains provide sites for interaction with C3b and factor B (Bb fragments), achieving tight binding and stability with C3 convertase. It binds to C3b and Bb through its TSP1-type 5 domain.
(Data source: Alphafold)
CFP Functions
CFP is a positive regulator of the complement system, primarily acting on the alternative pathway. Its functions are multifaceted, balancing immune defense and tissue protection.
Stabilization of the convertases: Stabilizing AP convertases, including C3 and C5 convertases. The TSR5 and TSR6 domains bind C3b and Bb, maintaining their binding and preventing dissociation. Competition with negative regulators (e.g., factor I, factor H) for convertase binding reduces sensitivity to inactivation. This stability extends the convertase half-life by 5-10 times, thereby enhancing C3b deposition and amplification. It plays a crucial role in anti-infection and the clearance of apoptotic cells.
Properdin, as a pattern recognition molecule for the complement alternative pathway, can directly bind to specific surfaces and recruit C3b or C3 ( H2O ) to de novo assemble functional C3 convertases (C3b , Bb or C3 (H2O), Bb), thereby independently initiating and amplifying the complement activation cascade. This process is strictly confined to the local microenvironment, and through the transient nature of its binding ability and the regulation by serum inhibitory factors, it effectively avoids systemic complement overactivation damage while driving local inflammatory effects (such as opsonization, MAC formation, and anaphylactic toxin release).

(Data source: Cortes C, et al. Front Immunol. 2013)
Targeted therapy for CFP
NM5072 is a monoclonal antibody targeting CFP, developed by NovelMed Therapeutics for the treatment of paroxysmal nocturnal hemoglobinuria (PNH), and has received Orphan Drug Designation (ODD) from the US FDA. It selectively blocks disease-related portions of the immune system, thereby inhibiting the bypass pathway cascade. Blocking Properdin function is expected to prevent the lysis of PNH erythrocytes, which may be related to the pathophysiology of PNH anemia. This monoclonal antibody could serve as a potent blocker of AP without affecting the parts of the immune system needed to fight infection. NM5072 has successfully completed a Phase 1 trial in healthy volunteers and met the expected safety profile. This potent drug is in various regulatory phases of development. Due to its longer half-life, the anti-Properdin molecule is a groundbreaking biological molecule.
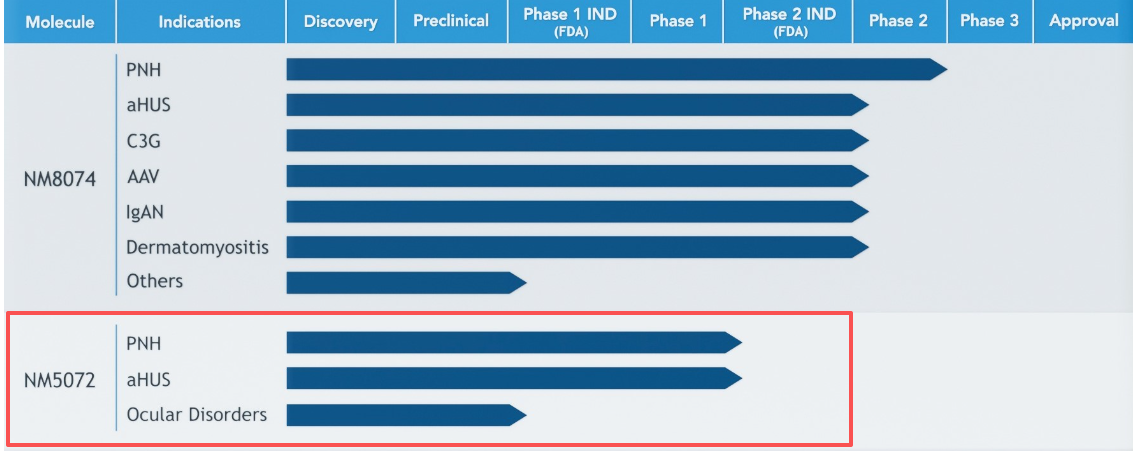
(Data source: NovelMed Therapeutics official website)
Tarperprumig (ALXN1820) is a bispecific VHH antibody targeting factors P. Originally developed by Alexion and later acquired by AstraZeneca, it is primarily used to treat diseases caused by dysregulation of the complement alternative pathway. Studies have shown that targeting factor P can inhibit the C3 complement pathway without affecting opsonization of bacteria, thereby reducing the likelihood of infection in patients. ANCA-associated vasculitis (AAV) is a rare inflammatory vascular disease caused by overactivation of the C5a complement pathway, which further activates neutrophils, leading to systemic inflammation and small vessel damage. Tarperprumig, by inhibiting the activity of the complement alternative pathway, holds promise for reducing inflammation and vascular damage.
