BTLA, also known as CD272, is a B and T lymphocyte attenuator. The BTLA protein belongs to the CD28 immunoglobulin superfamily (IgSF). BTLA recognizes the ligand HVEM/CD270 and is an inhibitory immune checkpoint, playing a dual role in regulating immune responses. Blocking BTLA's inhibitory effects can enhance immune responses to antigenic stimulation. The BTLA/HVEM axis may serve as a target for future cancer immunotherapy.
Expression and function of BTLA
BTLA is primarily expressed on the surface of lymphocytes and myeloid cells, including CD4+ and CD8+ T cells, NK cells, B cells, dendritic cells (DCs), and macrophages. Tissue distribution shows high levels of BTLA expression in lymph nodes, thymus, and small intestine.
B cells: BTLA-HVEM signaling inhibits B cell proliferation and secretion of specific cytokines (IL-6, IL-10, and TNFα), but does not affect the secretion of chemokines (IL-8 andMIP-1β).
T cells: BTLA not only plays a role in the early stages of T cell activation but also regulates T cell survival during T cell-mediated inflammation. BTLA-deficient T cells exhibit reduced apoptosis, thereby mediating sustained inflammation. BTLA also contributes to the induction of peripheral tolerance in CD4+ and CD8+ T cells.
DCs: Overexpression of BTLA can inhibit DC maturation and promote immune tolerance in immature DCs. Furthermore, the BTLA-HVEM pathway is involved in regulating DC homeostasis. BTLA+ DCs promote Foxp3 expression in T cells by upregulating CD5, leading to Treg cell differentiation and the induction of peripheral Treg cell tolerance.

(Data source: Andrzejczak A, et al. Biomark Res. 2024)
Structure and ligands of BTLA
The BTLA gene is located in an inverted position on chromosome 3q13.2 and encodes a type I transmembrane glycoprotein (33 kDa) consisting of 289 amino acids. The BTLA protein shares structural and functional similarities with PD-1 and CTLA-4. The BTLA protein consists of a signal peptide, an extracytoplasmic domain similar to IgC, a transmembrane domain, and an intracellular domain. The cytoplasmic region of BTLA contains three highly conserved tyrosine-containing motifs: a growth factor receptor-binding protein 2 (Grb2) binding site, an immunoreceptor tyrosine-based inhibitory motif (ITIM), and an immunoreceptor tyrosine-based switch motif (ITSM). A soluble form of the BTLA protein (sBTLA) is also generated through alternative RNA splicing.
BTLA is HVEM, which belongs to the tumor necrosis factor receptor (TNFR) superfamily. HVEM and BTLA interact in a 1:1 stoichiometric ratio, with the N-terminal cysteine-rich domain (CRD1) of HVEM interacting with the N-terminal extension of BTLA at positions 35-43.

(Data source: Wojciechowicz K, et al. Eur J Med Chem. 2024)
BTLA signaling pathway and regulation:
BTLA signaling negatively regulates immune responses by recruiting Src homology domain-containing phosphatases 1 and 2 (SHP-1 and SHP-2) mediated by two immunoreceptor tyrosine-based inhibitory (ITIM) motifs. BTLA interacts with HVEM in both cis and trans.
Trans-interaction: BTLA and HVEM are expressed on different cells. The BTLA-HVEM trans interaction provides bidirectional signaling for T cells. BTLA binding leads to the recruitment of SHP-1 and SHP-2, which downregulate TCR signaling and provide an inhibitory signal. Conversely, GRb-2 binding leads to activation of PI3K/Akt signaling, promoting T cell survival.
Cis-interaction: BTLA and HVEM are expressed on the same cells. BTLA-HVEM cis-interactions prevent BTLA or HVEM from interacting in trans with other co-signaling molecules and inhibit HVEM-dependent NF-κB activation in T cells, promoting tolerance.

(Data source Andrzejczak A, et al. Biomark Res. 2024)
The role of the BTLA/HVEM axis in cancer
In the context of cancer, the immunomodulatory functions of HVEM and its ligands are altered, particularly BTLA due to its inhibitory properties, leading to dysregulated anti-tumor immunity. In the context of cancer, enhanced BTLA expression on tumor-infiltrating lymphocytes increases trans interactions and leads to suppressed T cell-mediated anti-tumor responses.

(Data source: Sordo-Bahamonde C, et al. Mol Cancer. 2023)
Targeted therapy for BTLA
The BTLA/HVEM axis is a target for cancer immunotherapy. Multiple approaches are currently being developed at the preclinical and clinical levels, including monoclonal antibodies (monotherapy or in combination with anti- PD -1 or chemotherapy drugs) and sHVEM-producing CAR-T cell therapies.
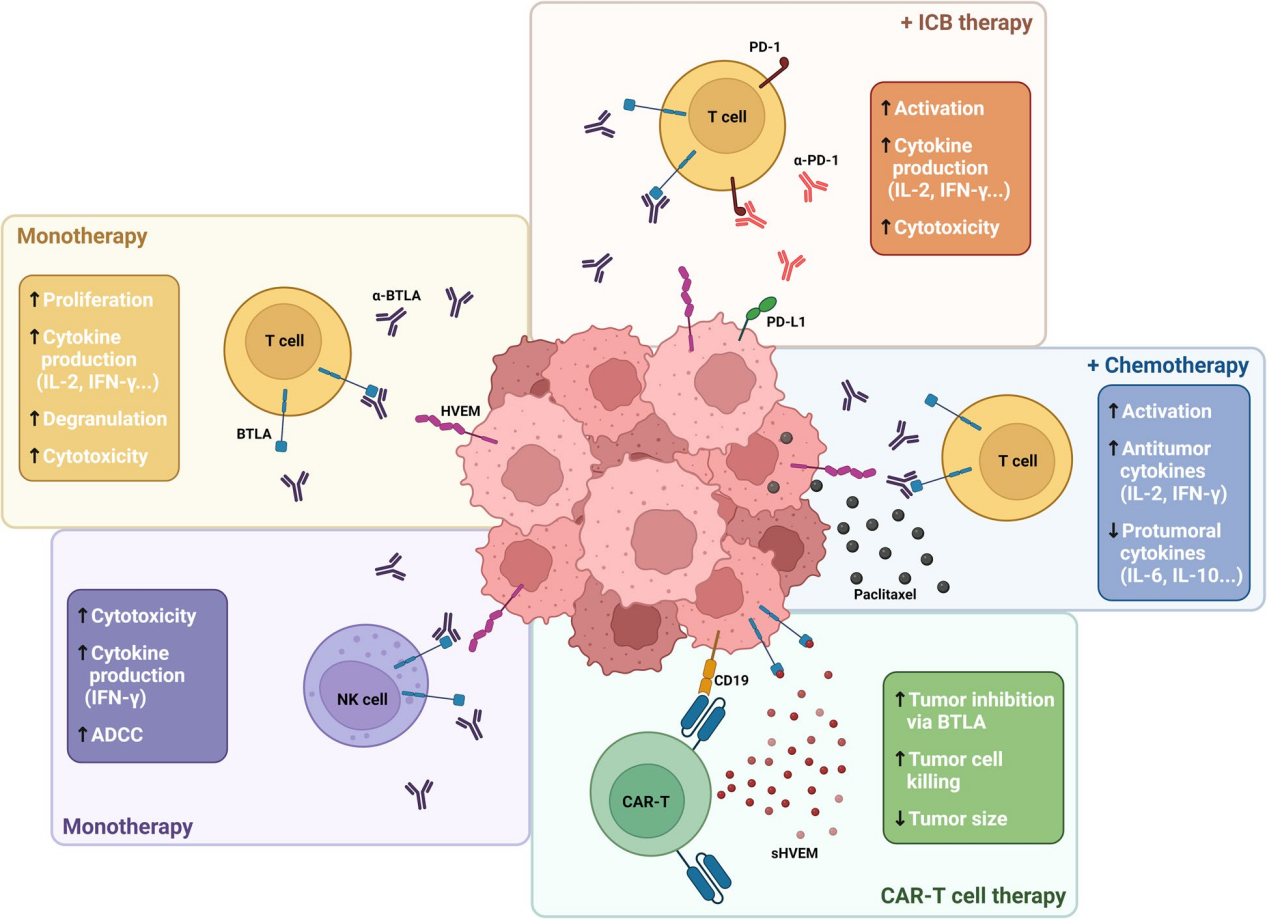
(Data source: Sordo-Bahamonde C, et al. Mol Cancer. 2023)
Tifcemalimab, a monoclonal antibody targeting BTLA, is being developed by Shanghai Junshi Biosciences Co., Ltd and is currently in Phase 3 clinical trials for the treatment of classical Hodgkin's lymphoma, refractory classical Hodgkin's lymphoma, and small cell lung cancer. Tifcemalimab has the potential to be combined with the anti-PD-1 monoclonal antibody toripalimab and is currently being extensively explored in various solid tumor and hematologic malignancies.
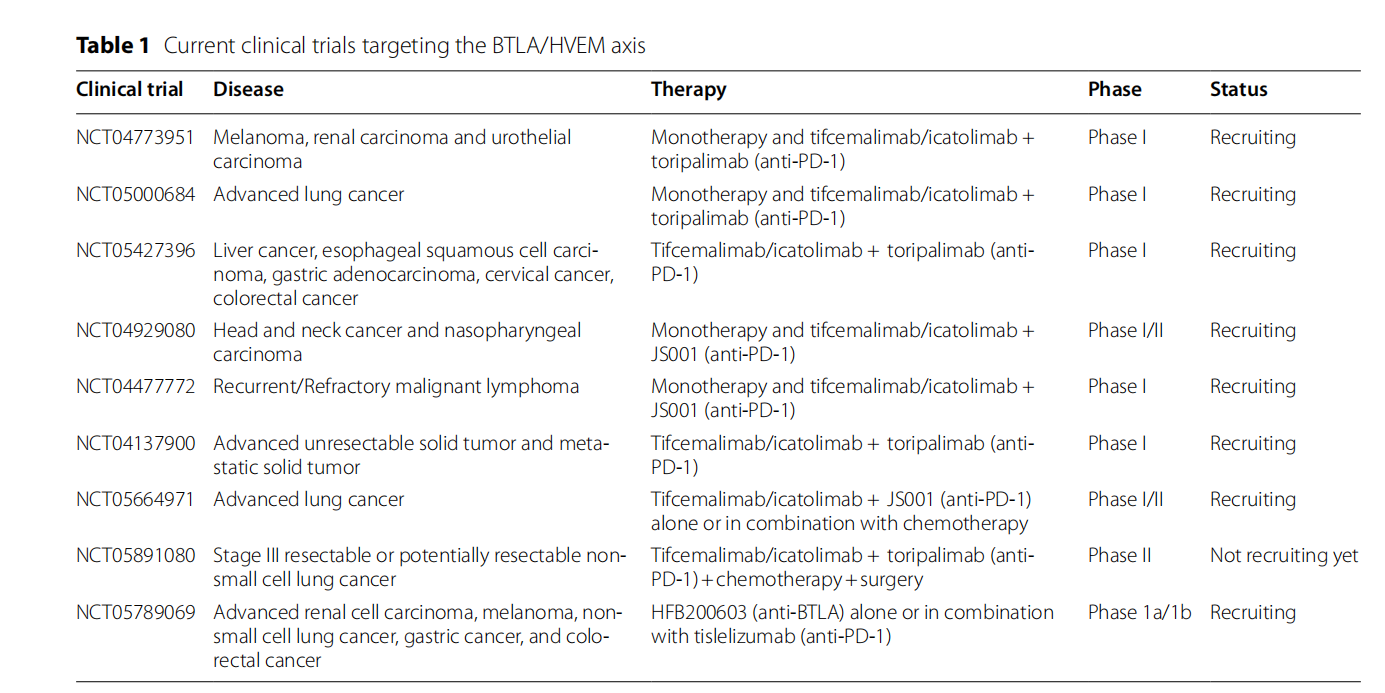
(Data source: Sordo-Bahamonde C, et al. Mol Cancer. 2023)
