Background
Tumor-infiltrating regulatory T cells (TI-Tregs) are key components of the tumor microenvironment (TME) that promote immune evasion and drive tumor progression. Although multiple therapies are currently available to eliminate TI-Treg cells, most have poor efficacy and carry the risk of inducing immune-related adverse events (irAEs). Recent studies have demonstrated that the CC chemokine receptor CCR8 is highly and specifically expressed on effector TI-Treg cells in both mice and humans, suggesting that CCR8 may be a promising target for selectively eliminating TI-Treg cells to treat various cancers.

On January 30, 2025, Professor Gao Qinglei's team from Tongji Hospital Tongji Medical College of HUST, published an article in Trends Immunol titled "CCR8: a promising therapeutic target against tumor-infiltrating regulatory T cells" This article reviews the latest research advances on CCR8 from the perspectives of its expression, function, and regulation, and summarizes the current status of CCR8-targeted therapies. These therapies have demonstrated promising efficacy and safety, representing an important category of next-generation putative cancer immunotherapies.

Expression of CCR8
CCR8 was first identified in monocytes and the thymus in 1997 and was subsequently shown to be expressed on CD4+ helper T ( Th ) 2 cells . In addition, CCR8 expression has also been detected on NK cells. Recently, single-cell RNA sequencing (scRNA-seq) analysis of human tumor samples revealed that CCR8 expression is specifically upregulated in TI-Treg cells in human breast cancer, colorectal cancer, and non-small cell lung cancer.

(Data source: Uniprot)
Structure of CCR8
CCR8 is a seven-transmembrane protein composed of seven transmembrane domains, an N-terminal extracellular region, and a C-terminal intracellular region. Within these seven transmembrane domains lie three extracellular loops (ECL1, ECL2, and ECL3) and three intracellular loops (ICL1, ICL2, and ICL3). ECL2 plays a key role in CCR8 ligand binding and serves as one of the primary sites of antibody recognition. The ICLs participate in receptor signaling and conformational changes. CCR8 is a G protein-coupled receptor (GPCR). CCR8 is activated by the endogenous C-C motif chemokine ligand 1 (CCL1) and couples to the inhibitory signaling protein G1. Binding of CCL1 to CCR8 triggers conformational changes in CCR8, leading to receptor binding of the α-subunit of the Gα protein (Gαi). The α subunit of the Gi protein forms polar and hydrophobic interactions with the transmembrane domain of CCR8 through its C-terminal α5 helix. Simultaneously, the β and γ subunits of the Gi protein (Gβ and Gγ) interact with the intracellular loop and C-terminal domain of CCR8, further stabilizing the complex. Binding of Gαi results in the replacement of its GDP with GTP, thereby activating Gαi, which in turn inhibits adenylate cyclase activity and reduces cAMP production, thereby altering intracellular signaling.

(Data source: Sun D, et al. Nat Commun. 2023)
Function of CCR8
Human CCR8 is a chemokine receptor with four ligands: CCL1, CCL8, CCL16, and CCL18. CCR8 is highly expressed on tumor-infiltrating T regulatory (TI-Treg) cells, and anti-CCR8 drugs can specifically target TI-Treg cells with high CCR8 expression. C-C chemokine ligand 1 ( CCL1 ) -CCR8 regulates Treg cell migration and recruitment into the tumor microenvironment (TME), influencing Treg cell immunosuppressive function in a STAT3 -dependent manner and promoting Treg cell survival through physical interactions with dendritic cells (DCs). CCL1-CCR8 on non-Treg CD4+ T cells can convert conventional T cells into Treg cells in a transforming growth factor ( TGF ) -β-dependent manner. NF-κB signaling mediated by the T cell receptor (TCR) and tumor necrosis factor receptor (TNFR) 2 can regulate CCR8 expression on Treg cells.

Progress in anti-CCR8 drugs
Because TI-eTreg cells express high levels of CCR8, anti-CCR8 agents can effectively target TI-Treg cells while minimizing the effects on Tconv and peripheral Treg cells. Anti-CCR8 agents can deplete eTreg cells, mitigate the exhaustion of CD8+ cytotoxic T lymphocytes (CTLs), promote the development of tumor-specific memory Tconv cells, activate dendritic cells (DCs), and induce less autoimmune responses than other strategies targeting Treg cells. A variety of CCR8-targeted therapies are under development, including monoclonal antibodies, antagonists, and bispecific antibodies.
Anti- CCR8 drugs can be combined with other cancer therapies . Anti-CCR8 monoclonal antibodies may enhance sensitivity to anti-PD-1 monoclonal antibodies by depleting Treg cells and converting cold tumors into hot tumors. Combining CCR8 antibodies with PD-1 or VEGF drugs can produce a synergistic anti-tumor effect without significant adverse reactions.
In a CT26 colorectal cancer mouse model, anti-CCR8 mAb treatment has been shown to promote the expansion of tumor-specific CD8+ T cells and induce a long-lasting memory response, significantly enhancing the therapeutic efficacy of PD-1 blockade. Approximately 15% of cancer patients treated with anti-PD-1 mAbs experience accelerated tumor growth, a phenomenon known as hyperprogressive disease (HPD), which is partly due to enhanced Treg cell activity and an imbalance in the ratio of Treg to Tconv cells. Simultaneous use of anti-CCR8 drugs to impair Treg cells may reduce HPD.
The anti-CCR8/CTLA-4 bispecific antibody GBD201 not only induces Treg cell clearance by enhancing ADCC but also inhibits Treg cell migration by blocking the CCL1-CCR8 signaling pathway. Compared to individual anti-CCR8 and CTLA-4 monoclonal antibodies, it exhibits complete blocking activity and potent binding properties, demonstrating similar or even superior in vivo tumor growth inhibition.
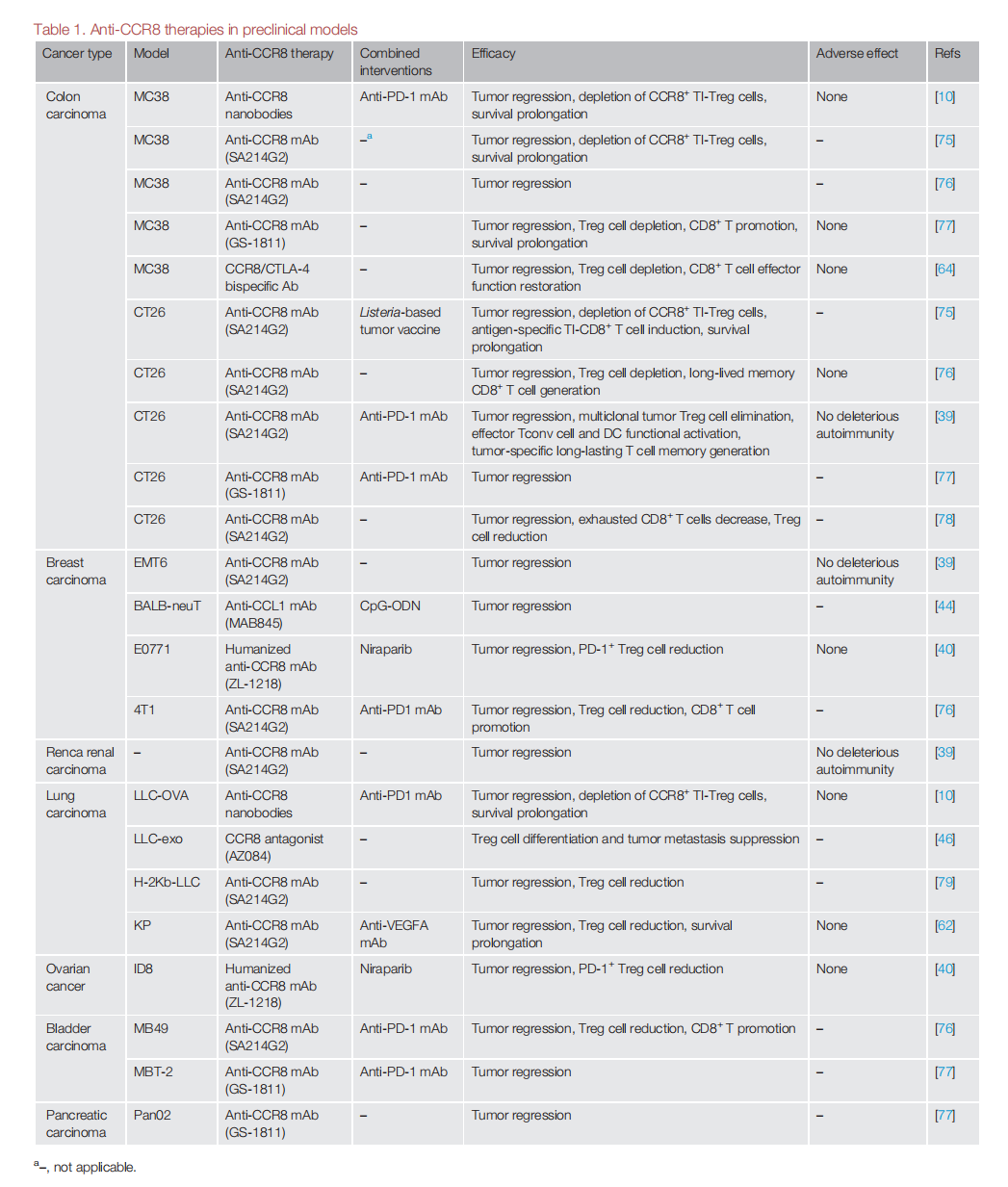
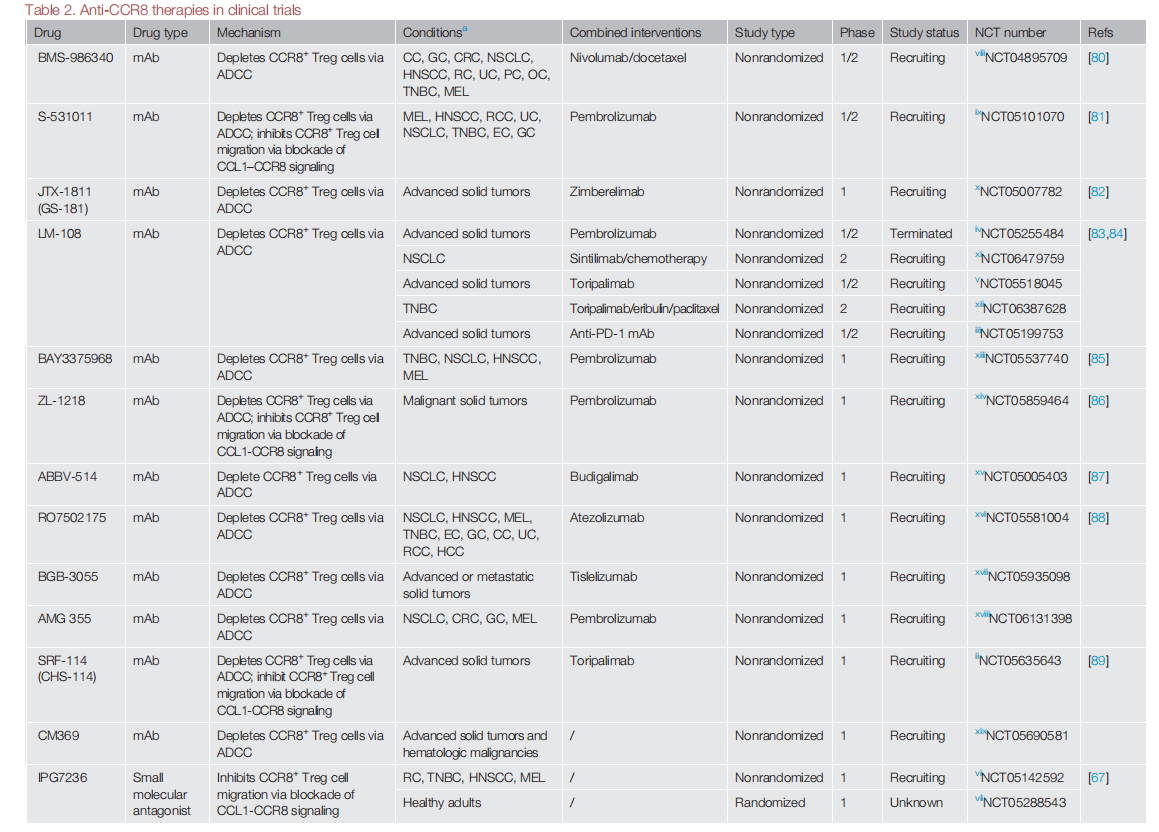
Many anti-CCR8 monoclonal antibodies have entered phase 1/2 clinical trials, and a phase 1 dose-escalation trial of SRF114 (an anti-CCR8 monoclonal antibody) (NCT05635643) recently reported preliminary results on the safety and robust effects of depleting CCR8+ Treg cells in 15 patients with advanced solid tumors.
A pooled analysis of results from three first-in-human, dose-escalation phase 1/2 trials of an anti -CCR8 monoclonal antibody (LM-108) (NCT05199753, NCT05255484, and NCT05518045) reported encouraging antitumor activity in 36 patients with gastric cancer when combined with an anti- PD -1 monoclonal antibody (ORR: 36.1% , p < 0.001 , disease control rate (DCR): 72.2%; median progression-free survival: 6.53 months ) , particularly in 11 patients who were resistant to anti -PD -1 monoclonal antibody therapy (ORR: 63.6%; DCR: 81.8%).
Summarize
CCR8 is highly expressed on TI-Tregs, and anti-CCR8 drugs have shown good anti-tumor efficacy while minimizing on-target/off-target toxicities. However, it also faces challenges. For example, targeting CCR8 as an anti-tumor treatment may produce some side effects, such as skin-related IRAEs, exacerbating ongoing autoimmune diseases. CCR8 expression varies in different tumor types, and multiple factors in the TME affect CCR8 expression. This suggests that we need to conduct a comprehensive assessment to evaluate the potential differential efficacy of anti-CCR8 therapy in different cancers. The clinical evaluation of anti-CCR8 drugs is still in its infancy and requires further evaluation in larger clinical trials. CCR8 has become a promising therapeutic target for TI-Treg cells in solid tumors, and anti-CCR8 drugs represent an important family of next-generation tumor immunotherapy.
