CD37 is a tetraspanin protein prominently expressed on the surface of B cells. It plays a crucial role in regulating B cell survival, shaping immune responses, and facilitating immune evasion. CD37 is highly expressed in acute myeloid leukemia (AML) and exhibits unique internalization properties compared to normal blood cells. CD37 is an attractive molecular target for immunotherapy of B cell-derived lymphomas and leukemias. CD37 is a promising target for monoclonal antibodies and antibody-drug conjugates in B and T cell lymphomas.
Expression distribution of CD37
CD37 is mainly expressed in B cells and T cells, and is also expressed in other immune cells, such as plasma cells, NK cells, granulocytes, monocytes, macrophages, Hofbauer cells, Kupffer cells, dendritic cells, Langerhans cells, and erythroid cells.
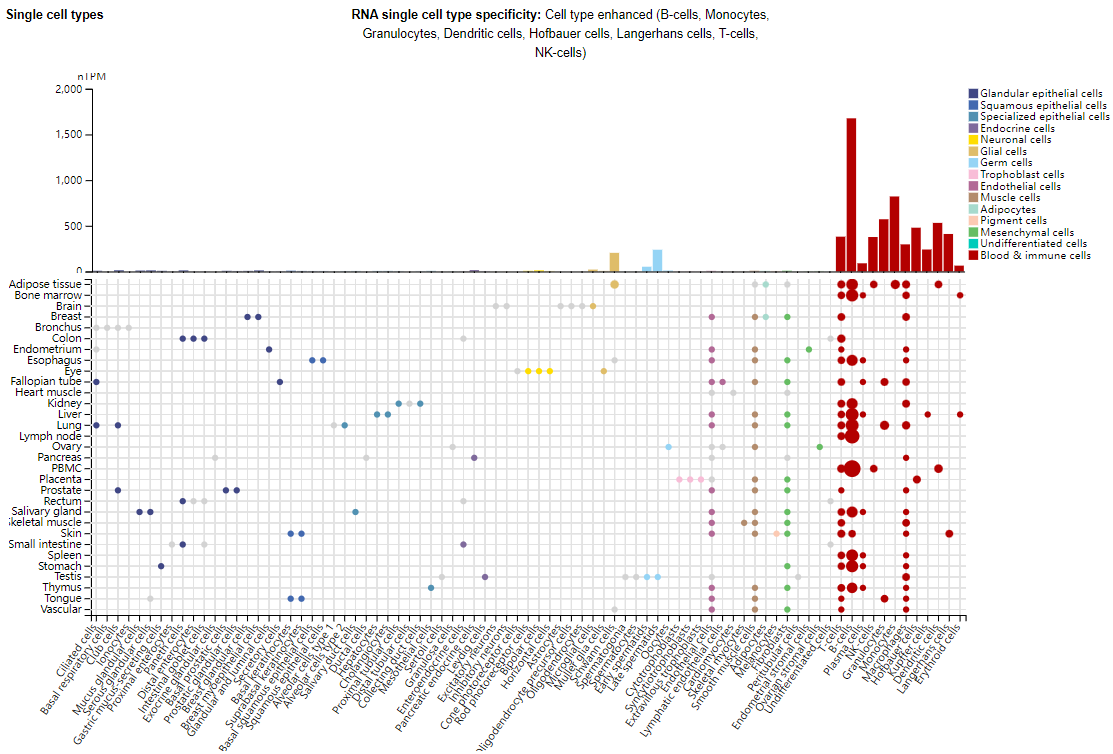
(Data source: Uniprot)
The structure of CD37 and its receptor
CD37 is a tetraspanin protein composed of 281 amino acids, including a short amino- and carboxyl-terminal tail, and four transmembrane regions (TM1, TM2, TM3, and TM4), separated by small intracellular and small and large extracellular loops. The conserved CCG motif (Cys-Cys-Gly sequence) on the large extracellular loop is a hallmark of tetraspanins.
The N-terminus of CD37 contains a weak immunotyrosine-based inhibitory motif (ITIM), while the C-terminus contains an immunotyrosine-based activation motif (ITAM). The phosphorylation state of these motifs determines whether CD37 activates or inhibits signal transduction within the cell.
Phosphorylation of ITIM recruits inhibitory signaling molecules such as SHP1, leading to cell apoptosis; while phosphorylation of ITAM activates survival signaling pathways such as PI3K/Akt, promoting cell survival.

(Data source: uniprot)
The role of CD37 in B cells
In B cells, CD37 activates two signaling pathways upon ligand binding. CD37 participates in both activating (PI3Kδ/p-Akt/p-GSK3/β-catenin) and inhibitory (SHP1/p-Akt/Foxo3 ; SOCS3/Jak) signaling pathways. CD37 plays a crucial role in pro-survival and pro-apoptotic signaling, with mechanisms closely linked to the PI3K/AKT pathway. Furthermore, CD37 regulates IL-6 receptor signaling through its interaction with SOCS3. On the one hand, CD37 mediates the recruitment of α4β1 integrin, thereby activating the PI3K/AKT pathway and promoting cell survival. On the other hand, CD37 forms a complex with SHP1, LYN, SYK, and PI3Kγ, leading to AKT inactivation and recruitment of SOCS3, thereby limiting the recycling of IL6/STAT3.

(Data source: Bobrowicz M, et al. Int J Mol Sci. 2020)
CD37-targeted therapy
The main anti-CD37 therapeutic approaches include monoclonal antibodies, antibody-drug conjugates, CAR-T cell therapy, and different tumor cell killing mechanisms have been developed, such as complement-dependent cell killing (CDCC), antibody-dependent cellular phagocytosis (ADCP), antibody-dependent cellular killing (ADCC), and direct induction of apoptosis.
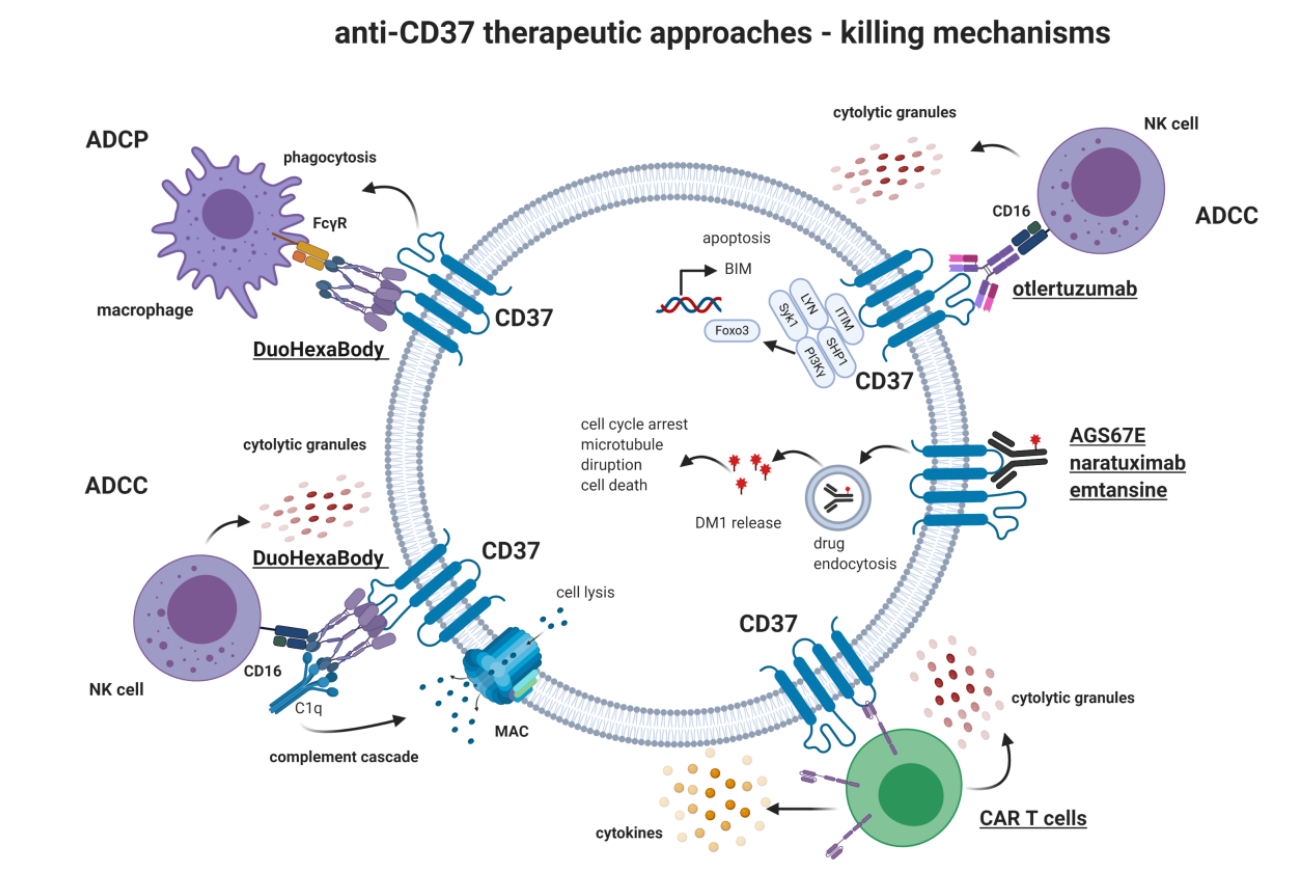
(Data source: Bobrowicz M, et al. Int J Mol Sci. 2020)
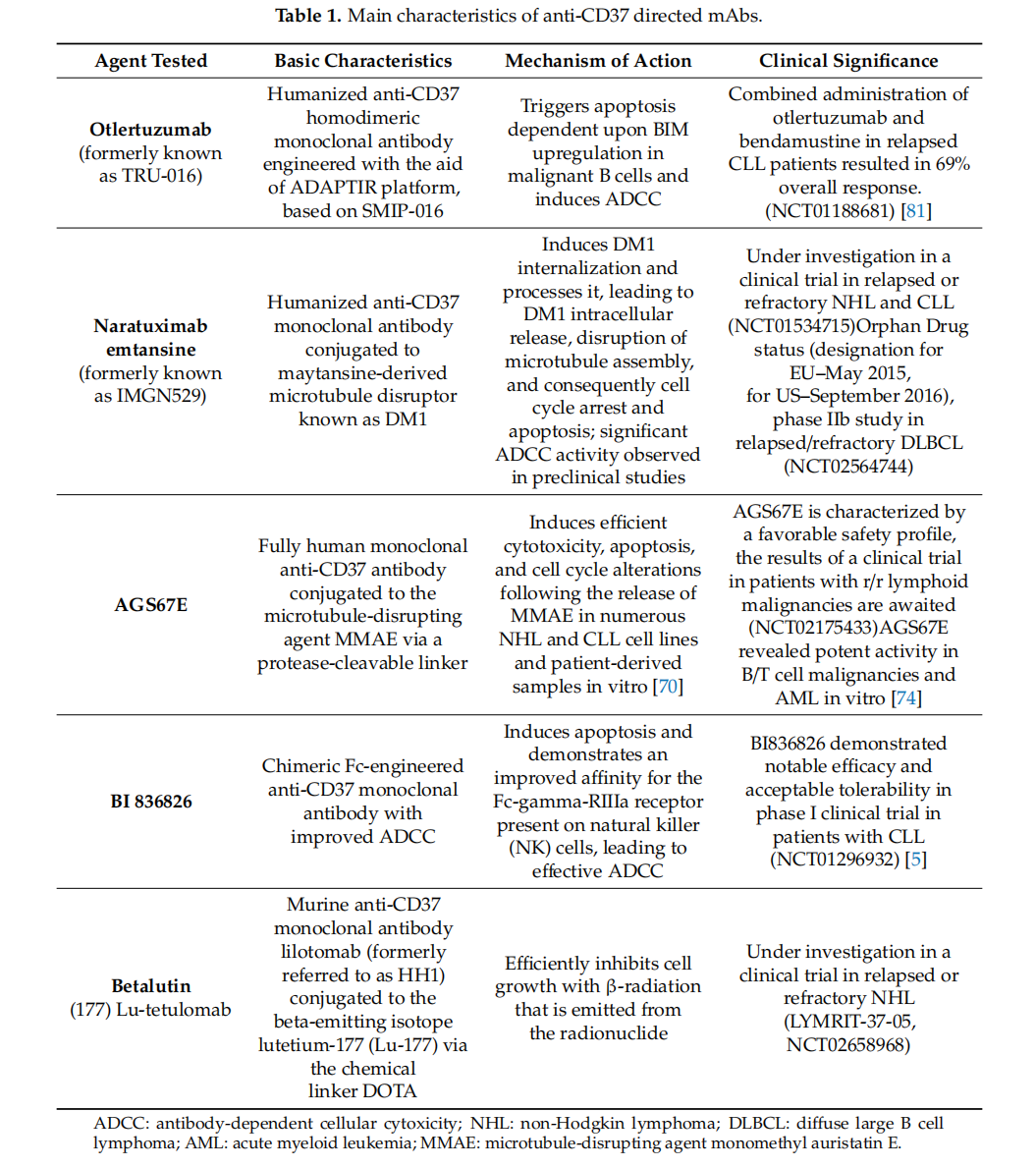
Antibody drugs targeting CD37 mainly include Otlertuzumab, Naratuximab emtansine, AGS67E, BI 836826 and Betalutin.
Otlertuzumab, a monoclonal antibody targeting CD37, was developed by Emergent BioSolutions and is currently in clinical trials for lymphoma and chronic lymphocytic leukemia (CLL). The otletuzumab clinical trial (NCT01188681) is a Phase 1b/2, open-label study evaluating the safety and efficacy of TRU-016 combined with bendamustine versus bendamustine alone in patients with relapsed CLL. In patients with relapsed CLL, the combination of otletuzumab and bendamustine resulted in an overall response rate of 69%.
Naratuximab emtansine ( MGN529) is an anti -CD37 antibody-drug conjugate (ADC) linked to the methadone DM1 . Upon binding of MGN529 to CD37, DM1 is internalized and released intracellularly, leading to microtubule disruption, cell cycle arrest, and apoptosis. In preclinical studies, naratuximab emtansine demonstrated high antitumor activity in CLL and CD37 -positive NHL models. It exhibited significant ADCC activity against lymphoma target cells and B-cell lymphoma cell lines .
AGS67E is a CD37-targeting ADC consisting of a fully humanized monoclonal IgG2 antibody linked to MMAE via a protease-cleavable linker. The drug induces potent cytotoxicity, apoptosis, and cell cycle changes in NHL and CLL cell lines and primary cells in vitro. This makes the antibody a promising new target for immunotherapy of B/T cell malignancies. Furthermore, AGS67E demonstrated antitumor activity in CLL and NHL xenografts, including rituximab-resistant cases. AGS67E has a favorable safety profile, and results from a clinical trial in patients with r/r lymphocytic malignancies are awaited (NCT02175433). AGS67E has demonstrated potent in vitro activity against B/T cell malignancies and AML , suggesting that it may represent a novel candidate for the treatment of AML.
CD37 CAR T cells specifically kill AML cells, secrete proinflammatory cytokines, and control cancer progression in vivo. Importantly, CD37 CAR T cells are not toxic to hematopoietic stem cells. Therefore, CD37 is a promising and safe CAR T cell target for AML.
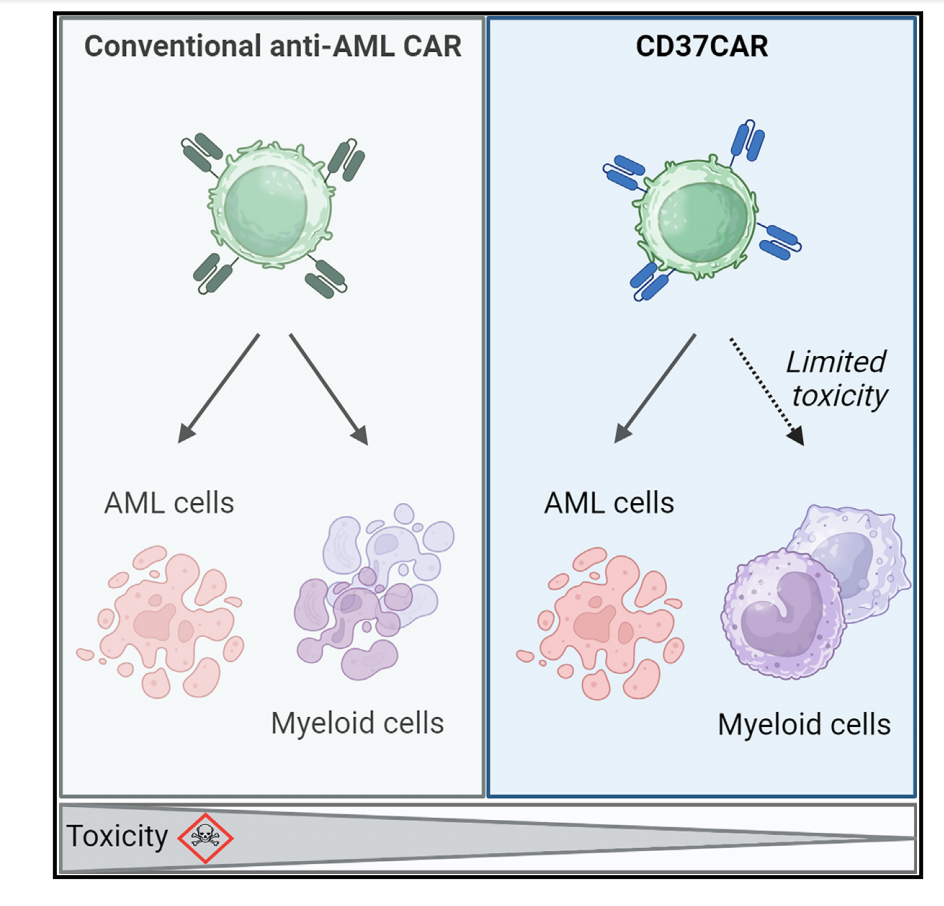
(Data source: Caulier B, et al. Cell Rep Med. 2024)
General Hospital Corp. has developed a CD37-targeted CAR-T cell therapy for the treatment of B-cell lymphomas, leukemias, and T-cell lymphomas. NCT04136275 is a Phase I clinical trial evaluating CAR-37 T cells in patients with relapsed or refractory CD37+ hematologic malignancies. This study is investigating the potential side effects of chimeric antigen receptor (CAR)-37 T cells (CAR-37 T cells) in patients with relapsed or refractory CD37+ hematologic malignancies.
