Epithelial cell adhesion molecule (EpCAM), also known as adenocarcinoma-associated antigen, epithelial glycoprotein (EGP), cell surface glycoprotein (TROP1), or M1S2, M4S1, MIC18, or TACSTD1, may function as a physical homology interaction molecule between intestinal epithelial cells and intraepithelial lymphocytes, providing an immune barrier within the mucosal epithelium and serving as the first line of defense against mucosal infection. EpCAM is involved in multiple aspects of cancer pathology, contributing to various carcinogenic characteristics, including epithelial-mesenchymal transition (EMT), hypoxic metabolism, stemness, angiogenesis, and immune evasion.

(Data source: Liu Y, et al. Exp Hematol Oncol. 2022)
EpCAM expression distribution
EpCAM is primarily expressed in proximal and distal enterocytes, Paneth cells, undifferentiated cells, and intestinal goblet cells. Under pathological conditions, EpCAM is widely expressed in cancer cells, including nearly all adenocarcinomas, including esophageal, gastric, colon, prostate, lung, ovarian, and breast cancers, and in malignant ascites. EpCAM has three expression patterns: in the cytoplasm , on the cell membrane, and at tight junctions. In tumors, it is primarily expressed at tight junctions.

(Data source: Uniprot)
The structure of EpCAM and its receptor
EpCAM is a transmembrane protein. The immature EpCAM molecule consists of 314 amino acids, including a signal peptide, which is cleaved during maturation. Mature EpCAM is composed of three domains: the extracellular domain (EpEX), the transmembrane domain (TM), and the intracellular domain (EpICD). EpEX is composed of a cysteine disulfide bonded N-terminal domain (ND), an A1 thyroglobulin (TY) domain formed by a cysteine-rich motif, and a C-terminal domain (CD). EpEX contains two epidermal growth factor (EGF)-like repeats followed by a cysteine-free region. Four cleavage sites (α, β, γ, and ε) within the EpCAM molecule allow for cleavage into soluble EpEX, the TM, and free EpICD. EpCAM can be proteolytically cleaved to produce a membrane-bound C-terminal fragment (EpCTF). EpCTF is then processed by γ-secretase into EpICD, a short 26-amino acid tail.

(Data source: Keller L, et al. Cell Stress. 2019)
EpCAM in cancer
In cancer, EpCAM on the membrane of cancer stem cells (CSCs) is cleaved by ADAM17 and γ-secretase, generating EpICD. Most EpICD is degraded by the proteasome, while the remaining EpICD can associate with FHL2, β-catenin, and Lef-1 to form a transnuclear complex that activates proliferation- and pluripotency-related genes. The EpEX/EGFR pathway and the EpICD transnuclear complex promote cell proliferation. EGF and TGF-β pathways regulate EMT markers and EpCAM expression. Under hypoxic conditions, EpCAM is upregulated in high ATP states, while HIF-1α is upregulated in low ATP states. HIF-1α-mediated CAIX overexpression occurs. A CAIX+ HCC subpopulation with elevated EpCAM and K19 expression exhibits enhanced resistance to chemoembolization. Furthermore, N-glycosylated EpCAM can regulate HIF-1α and promote EMT and stem cell-related properties. MHC-I/TCR interactions serve as T cell activation signals. Activated EpEX/EGFR/ERK pathway leads to reduced ubiquitination and degradation of PD-L1. PD-L1 on the tumor surface hinders the activation of CD8+ T cells, leading to immune escape.

(Data source: Liu Y, et al. Exp Hematol Oncol. 2022)
EpCAM -targeted therapy
EpCAM - targeting antibodies have been reported , including Catumaxomab, a bispecific T cell engager targeting EpCAM. It was approved for marketing in 2009 for the treatment of EpCAM-positive solid tumors, malignant ascites, and advanced gastric cancer. In February 2025, Lindis Biotech GmbH and Pharmavia announced that Catumaxomab had received marketing authorization from the European Commission, making it the only drug approved in Europe for the treatment of malignant ascites.
M701 is a bispecific T cell engager targeting EpCAM, developed by UZY for the treatment of advanced gastric cancer, advanced malignant solid tumors, colorectal cancer, and other diseases. A multicenter, open-label, dose-escalation study (NCT04501744) was initiated in October 2018 to evaluate the safety, tolerability, and PK/PD-R of a recombinant anti-EpCAM and anti-CD3 human-mouse chimeric bispecific antibody in malignant ascites via intraperitoneal infusion. An IND application was filed in China in 2016. A Phase 3 study (NCT06432296) in malignant tumors began in March 2024.

(Data source: YAbs)
CX-2051 is an antibody-drug conjugate (ADC) developed by Cytomx. CX-2051 is designed to open a therapeutic window for systemically administered anti-EpCAM ADCs. The drug candidate is currently in a Phase 1 dose-escalation study in patients with advanced solid tumors. This first-in-human study, CTMX-2051-101, aims to characterize the safety, tolerability, and anti-tumor activity of CX-2051 in adult participants with advanced solid tumors.

(Data source: Cytomx official website)
CX-2051 demonstrated promising efficacy in a Phase 1 trial, with an ORR of 28% and a DCR of 94%. The 10mg/kg dose achieved an ORR of 43% . Preliminary metastatic progression-free survival (mPFS) was 5.8 months, with 10 of 18 patients remaining on treatment, and no patients discontinued treatment due to side effects. CX-2051 was well tolerated, with no grade 4-5 side effects. Preliminary clinical data from CX-2051 demonstrate optimal efficacy in third-line and later-stage colorectal cancer treatment.

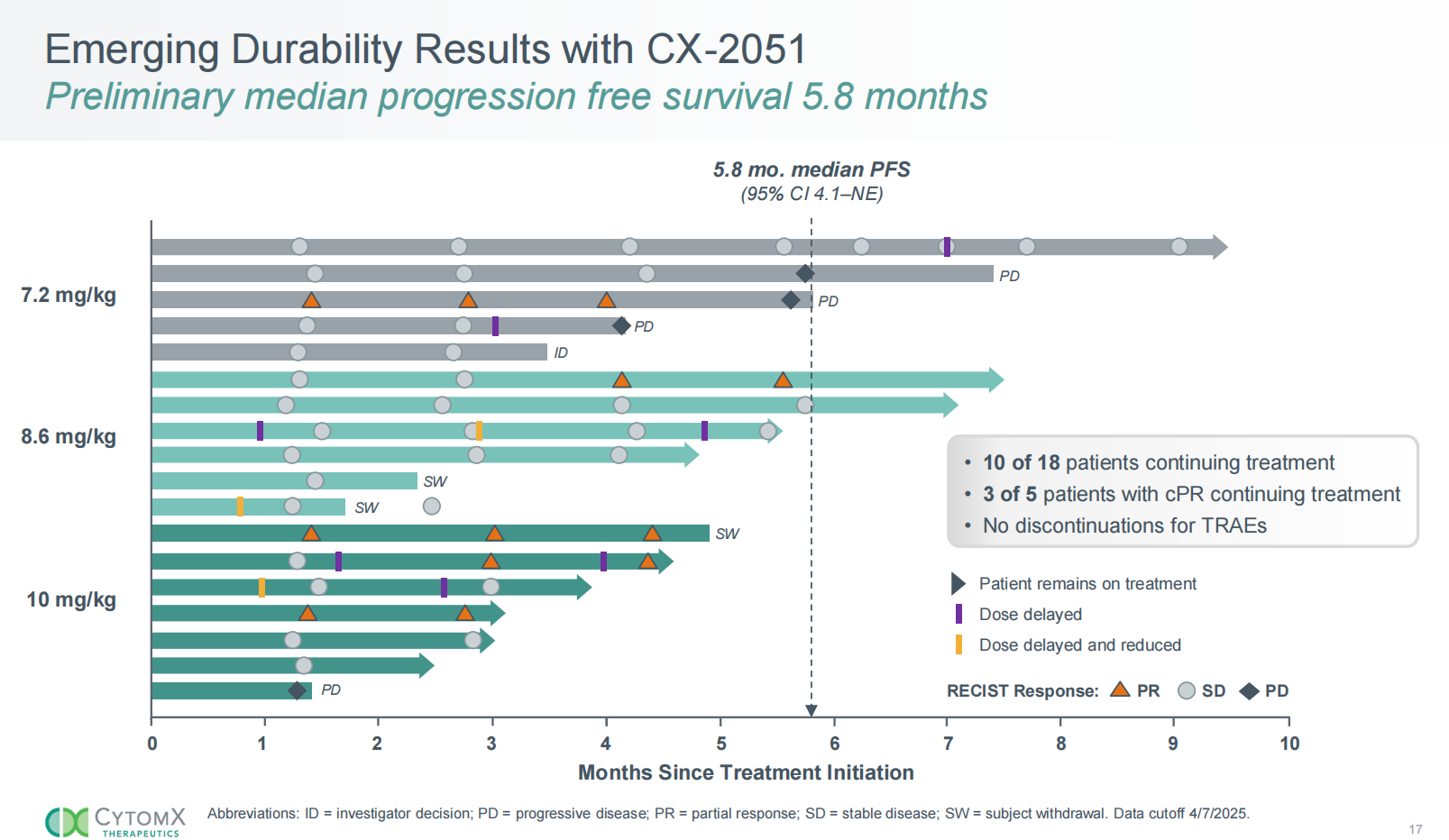
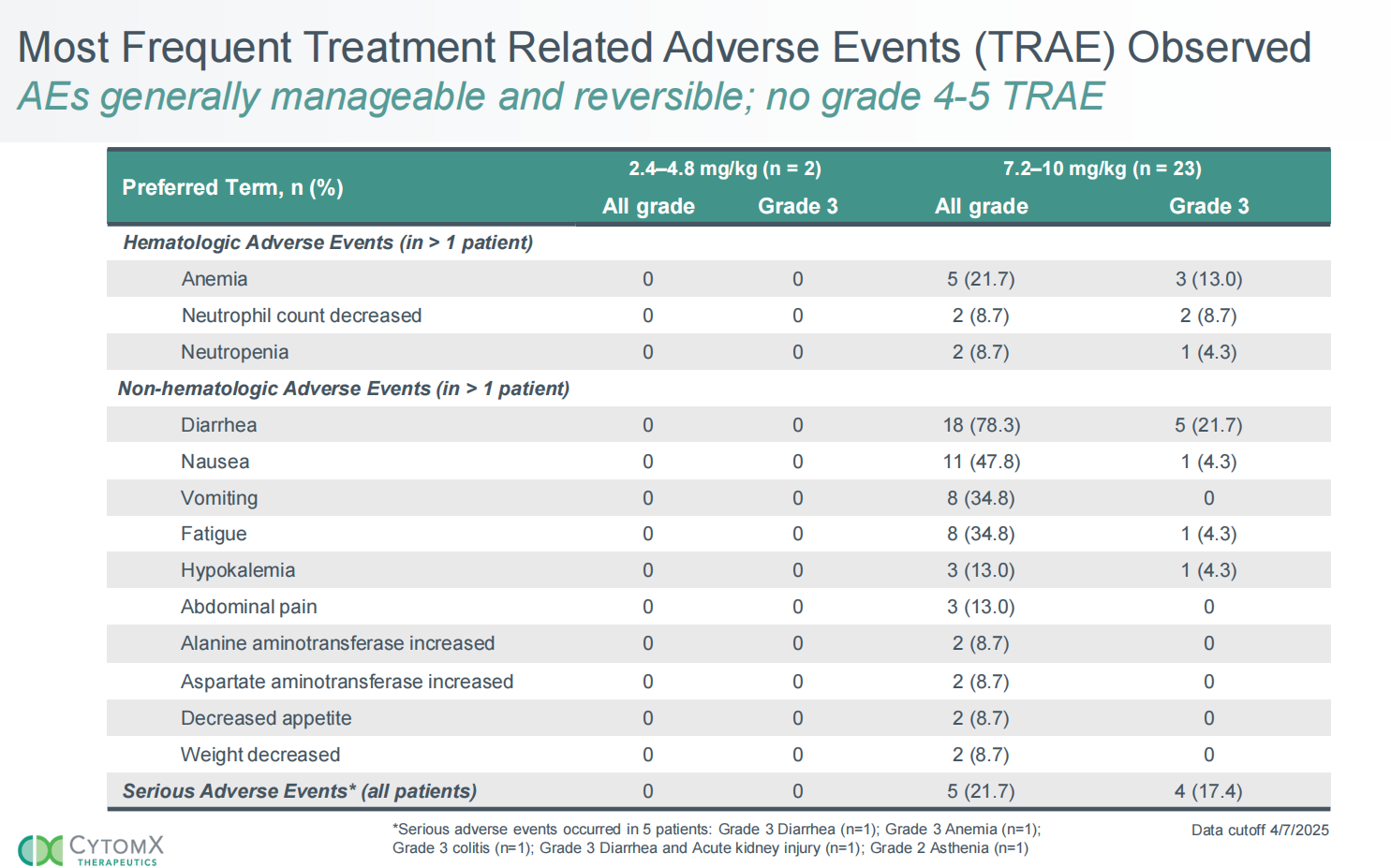
(Data source: Cytomx official website)
