C1s are an essential protein in the complement system and a component of the complement C1 complex. C1s are the initiator of the classical complement activation pathway. When the C1 complex binds to an antigen-binding immunoglobulin (IgG or IgM) complex, C1s is activated, leading to the formation of antigen-antibody complexes on the surface of pathogens. Because C1s is activated under various pathological conditions and is associated with inflammation, autoimmunity, and cancer development, it is becoming an informative biomarker for the diagnosis and treatment of a variety of diseases.

(Data source: Ye J, et al. Front Immunol. 2022)
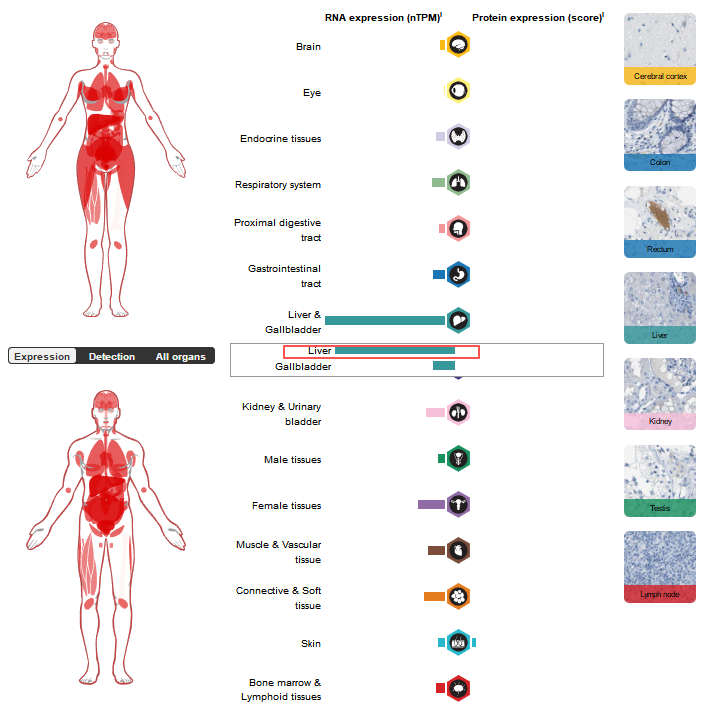
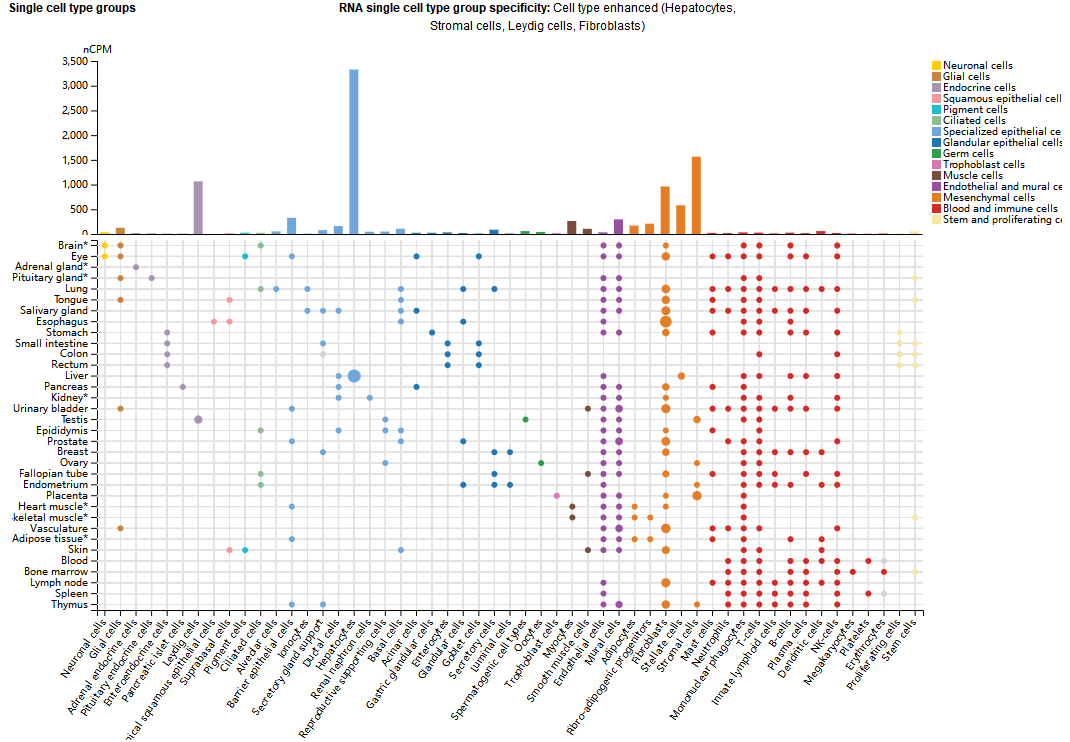
C1s expression distribution
At the tissue level, C1s are mainly expressed in the liver, while at the cellular level they are mainly expressed in hepatocytes, stromal cells, interstitial cells, and fibroblasts.
(Data source: uniprot)
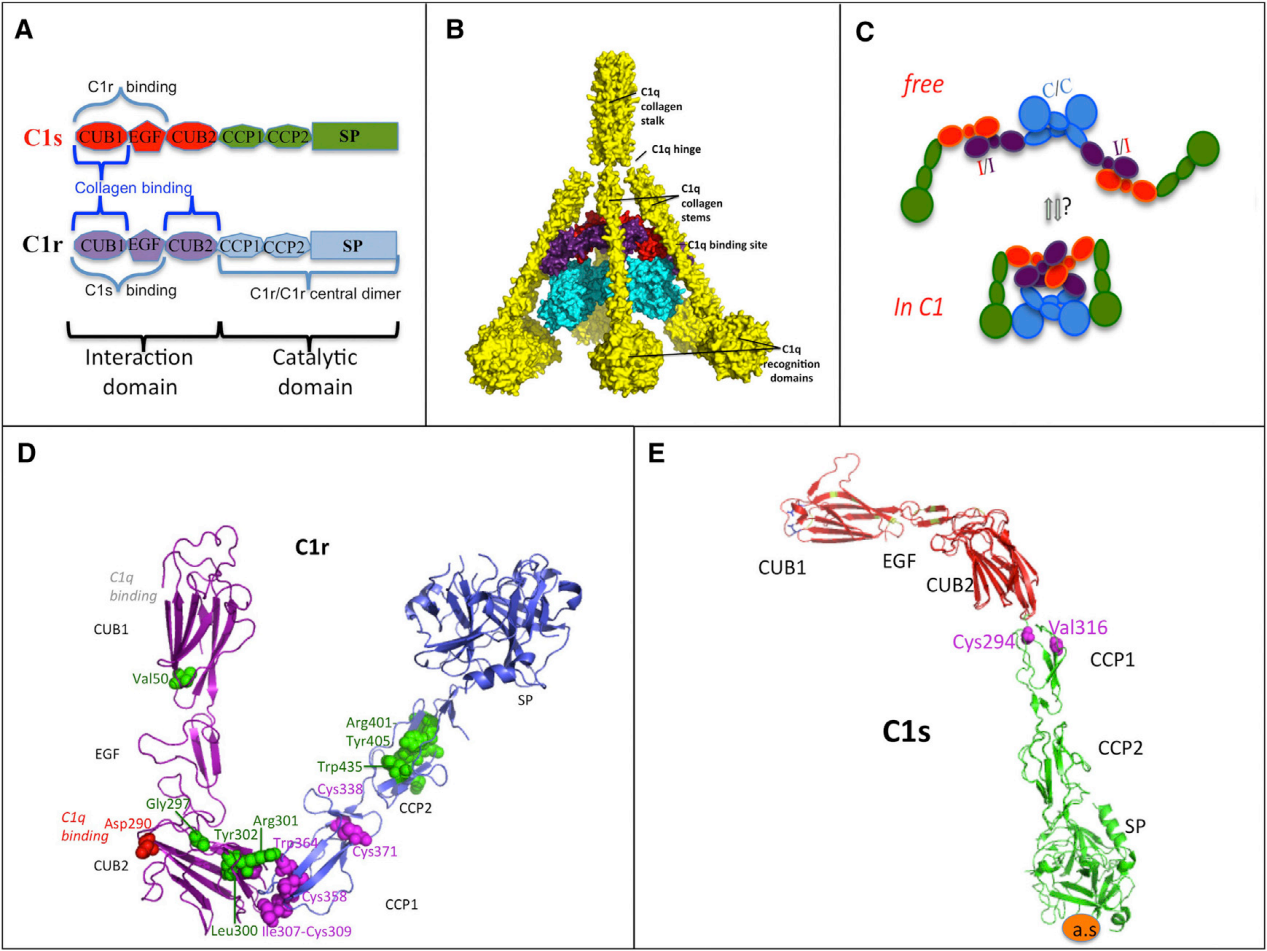
C1s structure
C1s is a secretory protein composed of 688 amino acids and consists of multiple domains, including the CUB, EGF, and CCP domains.
The CUB domain: The N-terminus contains two domains, CUB1 and CUB2, which are responsible for mediating the interaction between C1s and C1q and C1r, participating in the assembly of the C1 complex, and are crucial for the initiation of the classical pathway.
EGF-like domain:Located after the CUB domain, it participates in the stable binding between proteins and maintains the overall structural stability of the C1 complex.
The CCP modul: Contains two complement control protein modules, CCP1 and CCP2, which play a role in connecting and recognizing substrates, assisting C1s in recognizing complement components such as C4 and C2. The exosite in the CCP2 domain is a key region for substrate recognition, providing a specific target site for the design of selective inhibitors and avoiding interference with the function of other serine proteases.
(Data source: Kapferer-Seebacher I, et al. Am J Hum Genet. 2016)
The role of C1s in the complement system
C1s is a core protease initiating the classical complement pathway. Under physiological conditions, C1s forms the C1 complex with C1r and C1q, cycling in zymogen form. Activated C1s amplifies complement activation signals by specifically cleaving C4 and C2, driving the formation of downstream C3 convertase (C4b2a). C1s is a key link between adaptive immune recognition and innate immune effects (opsonization, inflammatory recruitment, and membrane attack), and is also an important drug target for treating various complement-mediated diseases.

(Data source: Hurler L, et al. Front Immunol. 2022)
C1s targeted therapy
Sutimlimab is a monoclonal antibody targeting C1s. It selectively inhibits the initial activation of classical CP by binding to and inhibiting C1s, while preserving the integrity of lectins and alternative pathways. On February 4, 2022, Sanofi announced that its complement C1s antibody, Sutimlimab, received FDA approval for the treatment of cold agglutinin disease (CAD), marketed as Enjaymo; its global rights were transferred to Recordati in late 2024. Positive results from the Phase III long-term extension study (Part B) of CADENNA were reported in 2024, confirming the sustained efficacy of Sutimlimab.

(Data source: Röth A, et al. Blood. 2022)
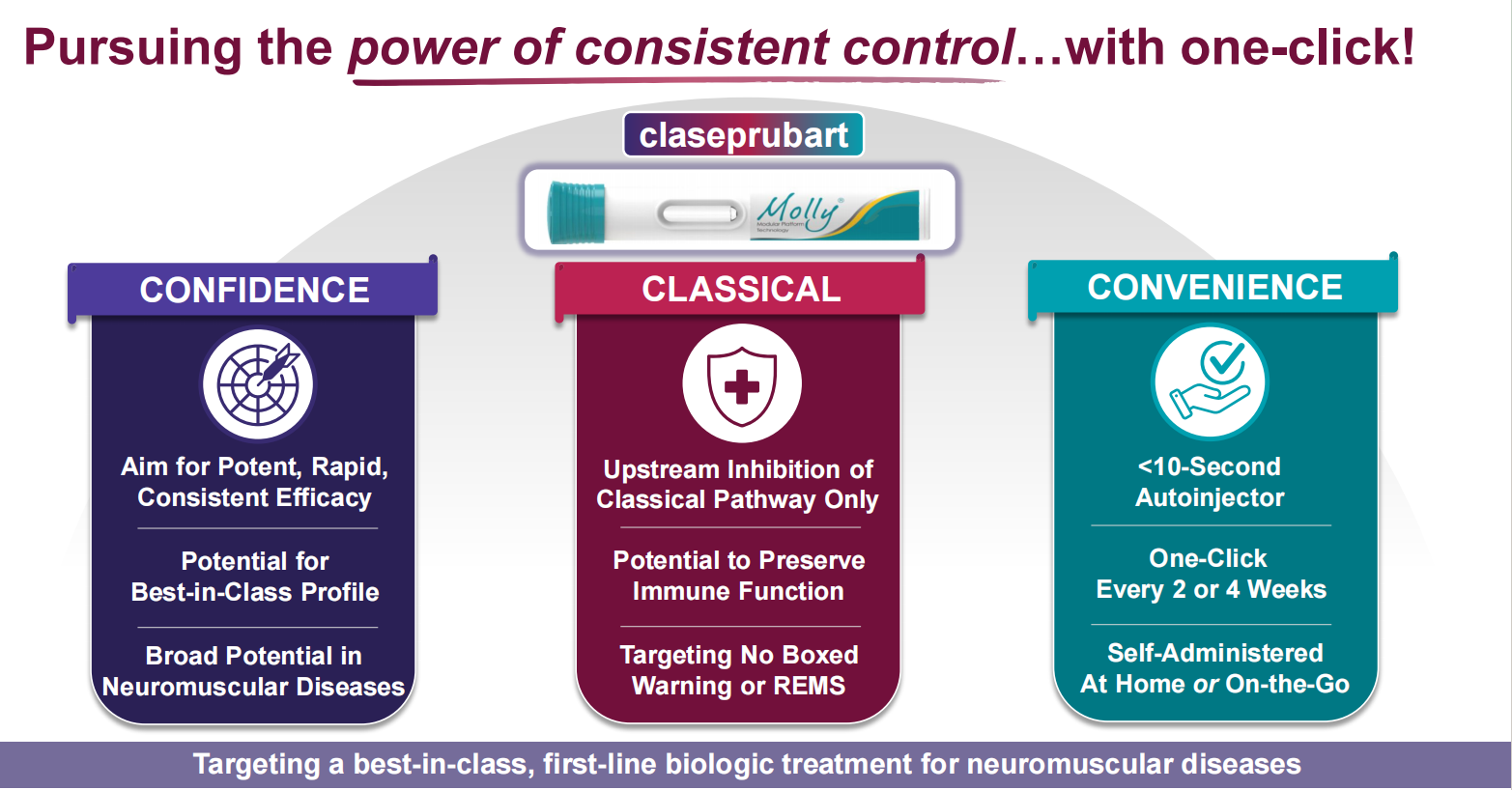
Claseprubart (DNTH103) is a potential top-tier complement inhibitor developed by Dianthus Therapeutics for the treatment of chronic inflammatory demyelinating polyneuropathy, multifocal motor neuropathy, and myasthenia gravis. It is engineered with an extended half-life, enhanced potency, and high selectivity, targeting only the active C1s complement protein that drives disease pathology. It is designed to be the first subcutaneous complement therapy that can be used very infrequently for patients with severe classic pathway-driven autoimmune diseases, requiring only a single dose every two weeks.
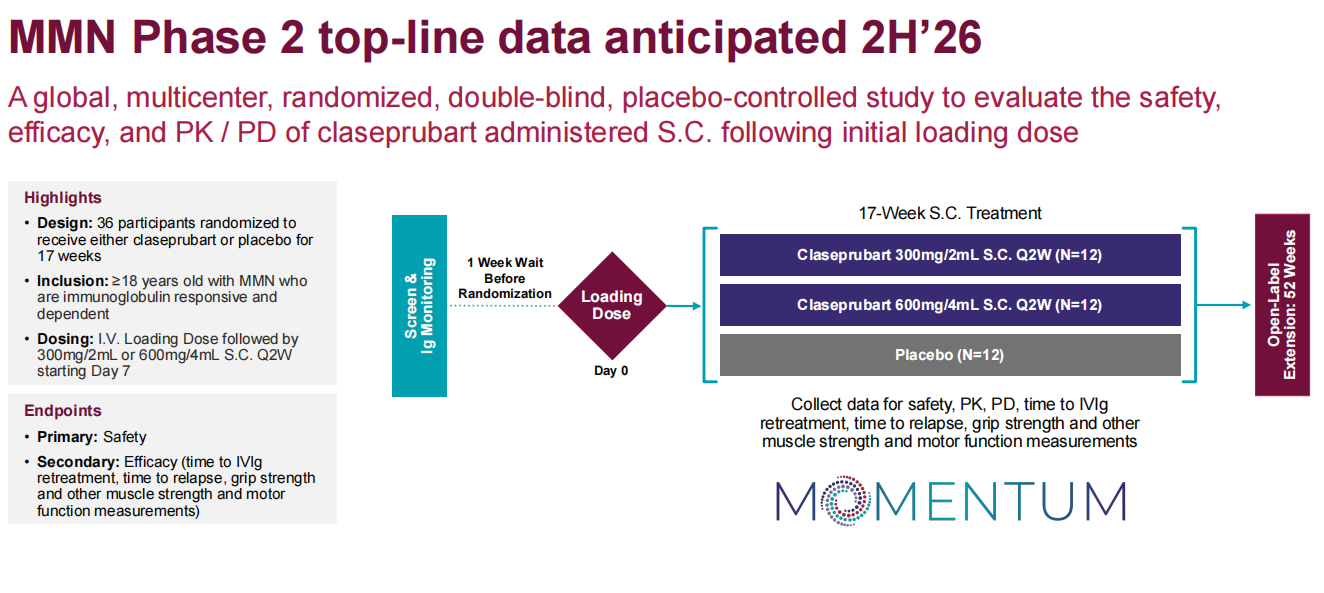
(Data source: Dianthus Therapeutics official website)
