Background
The Histidine tag (polyhistidine tag) is a commonly used protein tag used for protein expression, purification, and detection in biochemistry and molecular biology. It consists of a short peptide tag consisting of 6-10 histidine residues (commonly 6 His residues, i.e., 6His , HHHHHH ). The groups on the histidine residues form a stable metal chelate bond with metal ions (such as nickel or cobalt). Elution buffers containing low or no imidazole remove nonspecifically bound impurities, while elution buffers containing high imidazole concentrations (e.g., 250-500 mM) competitively displace the His tag from the metal ion, thereby eluting and collecting the target protein.

(Data source: BioRender)
Today's case study will combine the common problems encountered in our actual projects to explain the design and processing ideas of His-tag fusion proteins.
N-terminal or C-terminal
When the target protein needs to retain the N-terminal function (such as signal peptide, enzyme active center, N-terminal amino acid modification and other factors), it is recommended to place the His tag at the C-terminus of the target protein; when the C-terminus of the target protein is biased towards the functional domain segment, it is necessary to consider placing the His tag at the N-terminus of the target protein; if the above factors can be ignored, it is necessary to consider the exposure of the N/C-terminus of the tag addition (affecting subsequent purification).

(Case 1: Mabnus Bio original project data)
As shown in the figure of Case 1, the C-terminus of the target protein is the main functional domain, and the prokaryotic Escherichia coli expression system is selected for recombinant expression, When the His tag is placed at the C-terminus, due to the influence of overall hydrophobicity and structural folding, the His tag is not exposed and the amount of protein hanging on the column is very low. After adjusting the position of the His tag to the N-terminus, although the overall protein hanging on the column is not complete, the eluted protein content is significantly improved.

(Case 2: Mabnus Bio original project data)
In case 2, the extracellular segment 1-166aa was truncated as the target protein. Considering that there was a signal peptide at the N-terminus, the His tag was placed at the N-terminus, located between the signal peptide and the target protein, but this affected the normal expression of the target protein. When the His tag was placed at the C-terminus, the amino acids in the transmembrane segment close to the C-terminus were highly hydrophobic, which affected the tag hanging column. The amino acids near the membrane were further deleted and the 1-159 segment was selected as the target protein. The His tag was placed at the C-terminus, and both protein hanging column and yield were significantly improved (due to the influence of glycosylation at the amino acid site of the protein, the band was larger and formed a tail).

(Case 3: Mabnus Bio original project data)
Therefore, when designing the His tag position for recombinant expression and analyzing the results, we must first consider the influence of the target protein's own sequence factors. Secondly, when encountering the results in Case 3, where the expression test shows high abundance of the target protein but low column loading efficiency, we need to consider the impact of the His tag's non-exposed position. Furthermore, we can consider increasing the His tag length to improve column loading efficiency (for example, replacing 6His with 9His, 14His, etc.).
Purification
the natural histidine residues of the endogenous protein of the expression host , it is easy to cause nonspecific binding during the Ni resin purification process , resulting in a large amount of nonspecific impurities in the elution of the His-tagged fusion protein - Case 4.

(Case 4: Mabnus Bio original project data)
On the one hand, the purity can be improved by adjusting the binding time to reduce nonspecific protein adsorption - Case 5. If the size difference between the impurity protein and the target protein is significant, it can also be filtered out and recovered by selecting an ultrafiltration tube of appropriate size;

(Case 5: Mabnus Bio original project data)
On the other hand, secondary purification is used to further remove impurities - Case 6 (it should be noted that the sample needs to be dialyzed against imidazole before secondary purification to avoid affecting binding);
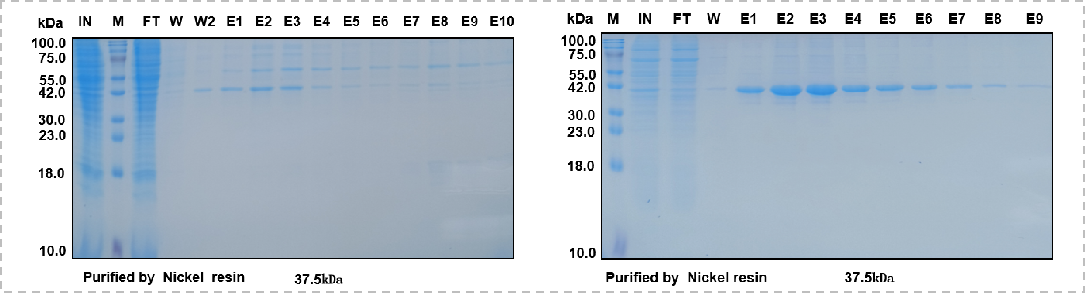
(Case 6: Mabnus Bio original project data)
In addition, consider increasing the imidazole concentration gradient (10mM, 20mM, 50mM, 100mM, etc.) during the wash stage to determine the optimal wash concentration, then wash the resin extensively at this concentration, or manually perform denaturation purification, followed by dialysis replacement of the conventional buffer for renaturation to enhance purification efficiency - Case 7 (This solution is suitable for situations where the protein itself has a large amount of supernatant expression and the protein is relatively stable).

(Case 7: Mabnus Bio original project data)
Inclusion bodies
At the same time, since the His tag has limited regulation of the hydrophilicity of highly hydrophobic amino acids, it is often encountered that the fusion protein exists in the form of inclusion bodies or is not expressed, making it difficult to obtain soluble supernatant protein through a single purification.
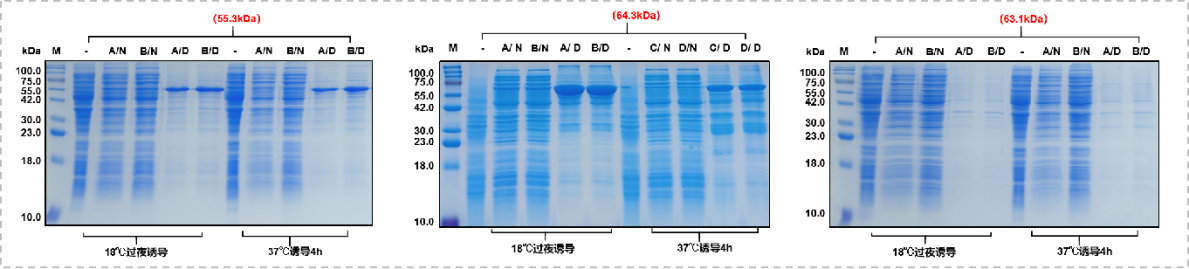
(Case 8: Mabnus Bio original project data)
To avoid the increased cycle time and costs associated with subsequent denaturation and purification, as well as unpredictable renaturation operations, consider timely adjustments to the solubility tag + His dual tag design to improve recombinant protein solubility and high-volume expression early on, increasing subsequent amplification yield and reducing costs (Case Studies 9 & 10 ). Alternatively, consider adding a restriction site between the tag and the target protein, and later remove the solubility tag or purification tag through enzymatic cleavage. This approach also typically imposes operational requirements and results in significant protein loss, requiring comprehensive consideration of multiple factors.

(Case 9: Mabnus Bio original project data)
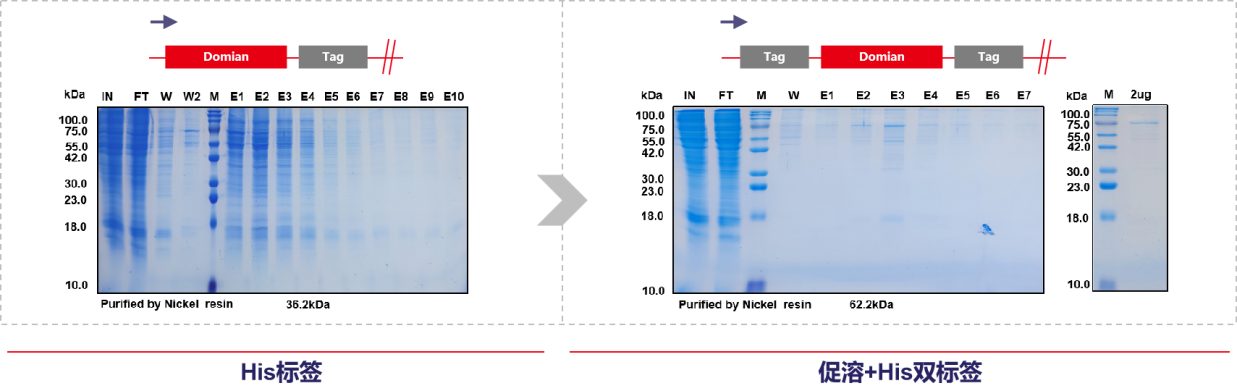
(Case 10: Mabnus Bio original project data)
Summary
Common issues we encounter during the expression and purification of His-tagged fusion proteins include: 1. low or no expression of the fusion protein; 2. low protein yield and purity after purification; 3. poor solubility of the fusion protein, resulting in the formation of inclusion bodies. We can effectively resolve most of these issues by optimizing expression conditions, purification parameters, and His-tag design. If you have any other questions, please contact us.
