BAIAP2 is an adaptor protein, also known as FLAF3 or IRSp53; it connects membrane-bound small G proteins to cytoplasmic effector proteins. It is essential for CDC42-mediated actin cytoskeleton remodeling and RAC1-mediated membrane folding. It participates in the regulation of the actin cytoskeleton by WASF family members and the Arp2/3 complex. It plays a role in neurite growth. It synergistically promotes the formation of filopodia with ENAH. It participates in actin cytoskeleton remodeling during bacterial infection. When associated with EPS8, it participates in actin bundle formation and promotes the protrusion of filopodia. Its dysfunction is directly associated with various mental illnesses, including autism and schizophrenia.
BAIAP2 expression distribution
BAIAP2 expression exhibits significant brain tissue specificity and developmental stage dependence, and is expressed in regions such as the cerebral cortex, hippocampus, striatum, and cerebellum. It is also expressed in peripheral tissues such as the spleen, lungs, liver, and testes. It is expressed in various cell types, primarily in apical squamous epithelial cells.
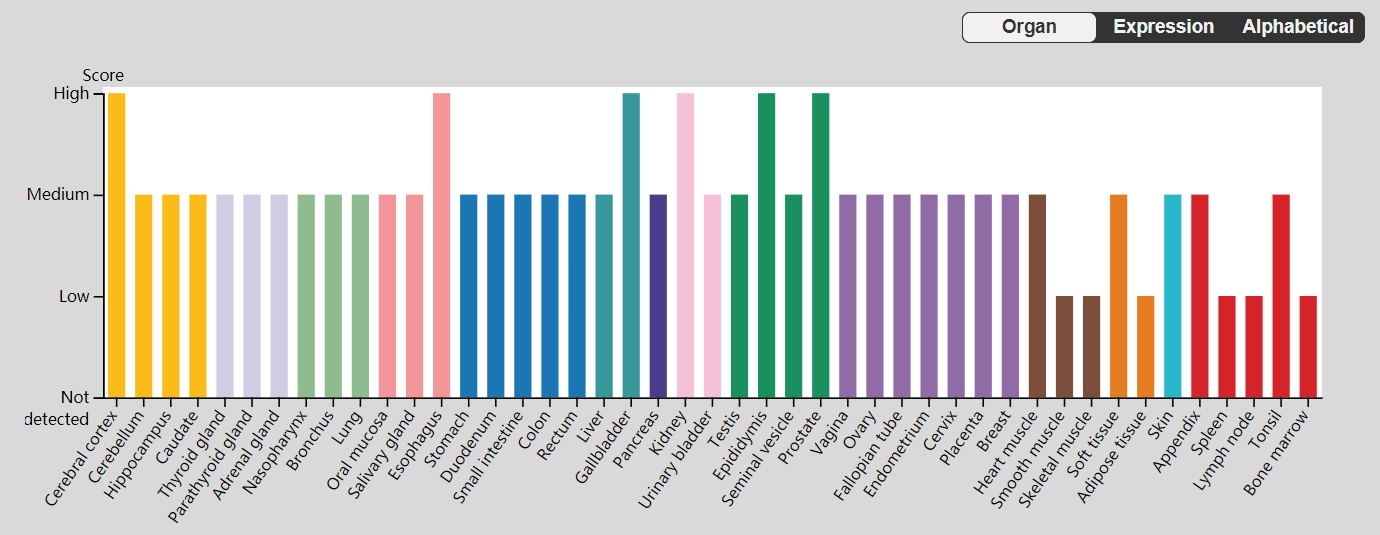
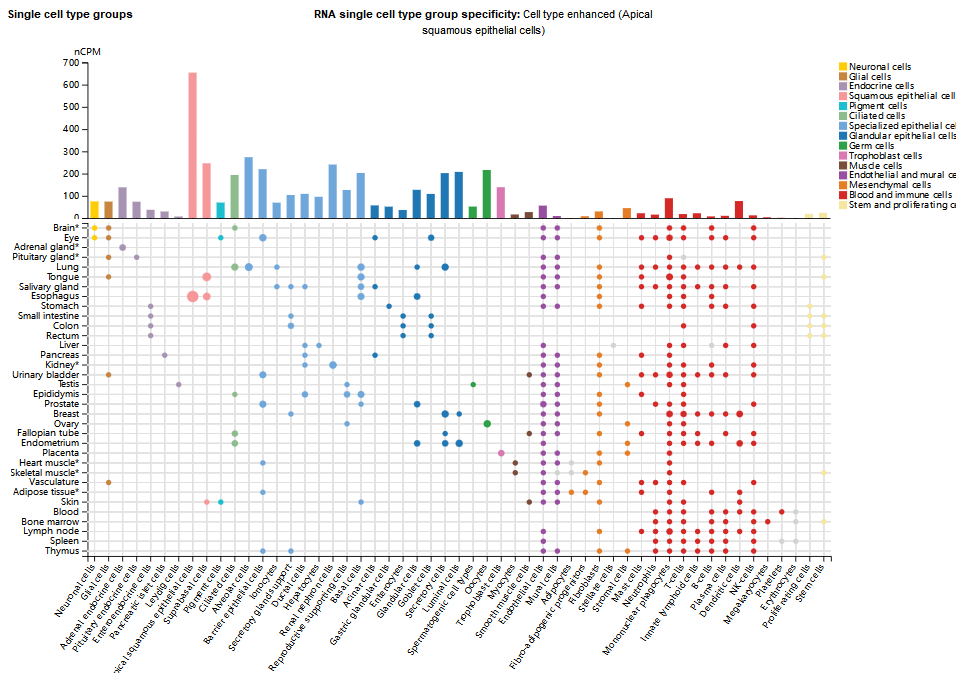
(Data source: uniprot)
The structure of BAIAP2 and its receptor
The BAIAP2 gene is located on human chromosome 17q25. The IRSp53 protein contains multiple protein-protein interaction domains, including the IMD (IRSp53-MIM homology domain; also known as I-BAR), CRIB-PR (CDC42/Rac interaction binding proline-rich) domain, SH3 (Src homology 3) domain, WW domain, WH2 (WASP homology 2) domain, and the C-terminal PDZ-B domain (PSD-95/Dlg/ZO-1 domain binding) motif.
The IMD/I-BAR domain: Located at the N-terminus, forms an anti-propeller-shaped dimer with a curved, positively charged surface. It can sense and induce the cell membrane to bulge outward ( negative membrane curvature), and is key to initiating membrane protrusions such as filopodia.
SH3 domain: Located at the C-terminus, the typical SH3 barrel fold contains a groove that recognizes and binds to proline-rich (PxxP) sequences on downstream effector proteins, and is responsible for recruiting actin regulatory complexes.
The head-to-head dimer of IRSp53 self-inhibits in a closed conformation through intramolecular interactions involving the CRIB-PR and SH3 domains. Activated (GTP-bound) CDC42 binds to the CRIB-PR domain, opening the protein and allowing the SH3 domain to bind downstream effectors such as N-WASP and WAVE2.

(Data source: de Groot JC, et al. Structure. 2011)
BAIAP2 regulates dendritic spine development
Dendritic spines are tiny, spiny projections on the dendritic branches of neurons, serving as core structural units in the brain that receive and process excitatory synaptic signals. BAIAP2/IRSp53 acts as a core scaffold in the postsynaptic density. Activated Cdc42 binds to the N-terminal CRIB-PR domain of BAIAP2, releasing its autoinhibition and activating it. Activated BAIAP2 drives local membrane bulging through its IMD domain. Simultaneously, it recruits actin regulators such as the WAVE complex and Eps8 through its C-terminal SH3 domain, converting Rac1/Cdc42 signals into local actin polymerization, driving spine extension and maturation. During development, BAIAP2 expression levels increase synchronously with dendritic spine density; knockout or mutation leads to significantly reduced spine density, abnormal head morphology, and impaired synaptic transmission. IRSp53 deficiency enhances NMDA receptor function, causing abnormal synaptic plasticity, manifested as increased LTP but impaired learning and memory, suggesting its fine-tuning role in excitatory synaptic homeostasis.

(Data source) Kang J, et al. Neuropharmacology. 2016)
The Relationship Between BAIAP2 and Disease
The IRSp53/BAIAP2 protein is associated with normal human brain function and a variety of mental disorders, including autism spectrum disorder, schizophrenia, and attention deficit hyperactivity disorder.
In healthy individuals, a common single nucleotide polymorphism (SNP) variant on the BAIAP2 gene is associated with emotional regulation of human memory strength. In individuals with autism and schizophrenia, multiple SNPs are associated with disease susceptibility. Furthermore, in individuals with autism, a novel copy number variant (deletion) resulting in the deletion of the entire IRSp53 gene has been found. In patients with schizophrenia, neologism missense and nonsense mutations in the IRSp53 gene lead to amino acid substitutions in the F486L protein following the WW domain and truncation of the protein within the IMD domain, respectively.
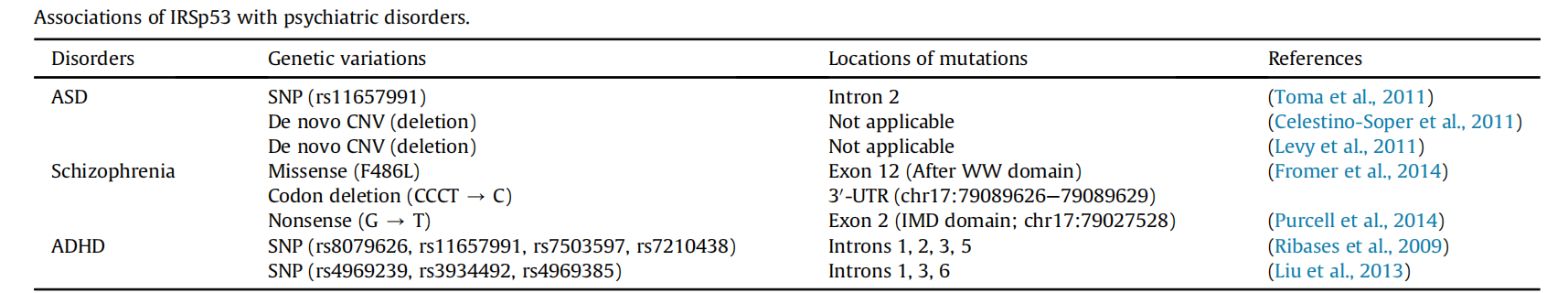
(Data source: Kang J, et al.) Neuropharmacology. 2016)
In studies of Japanese encephalitis virus (JEV) infection, BAIAP2-positive neurons were found to be highly susceptible to JEV. The study showed that after JEV infection, the expression of virus-related genes in BAIAP2-positive neurons was significantly increased, and the number of neurons decreased with increasing infection severity. This suggests that BAIAP2 may serve as a target for JEV invasion of neurons, participating in virus-induced neurological damage and inflammatory responses, and providing a potential target for developing therapeutic strategies against viral encephalitis.

(Data source: Yang L, et al.) J Neuroinflammation. 2024)
