Vascular adhesion protein 1 (VAP1), also known as saminoglycan-sensitive amine oxidase (SSAO) or copper-containing amine oxidase 3 (AOC3), is located on the surface of endothelial cells. It also acts as a cell adhesion protein, mediating the binding of lymphocytes to peripheral lymph node vascular endothelial cells and participating in lymphocyte exudation and recirculation in an L-selection-independent manner. It plays a central role in inflammatory responses, vascular diseases, and fibrotic processes, and has become a highly promising therapeutic target for various chronic diseases.

(Data source: Bhattacharjee P, et al. Med Res Rev. 2025)
VAP1 expression distribution
VAP-1 is widely expressed in the vascular system in the human body. It is mainly located on the surface of vascular smooth muscle cells and endothelial cells, with particularly significant expression levels in highly vascularized tissues such as hyperendothelial venules in lymph nodes, hepatic sinusoidal endothelium, lungs, small intestine, and appendix.
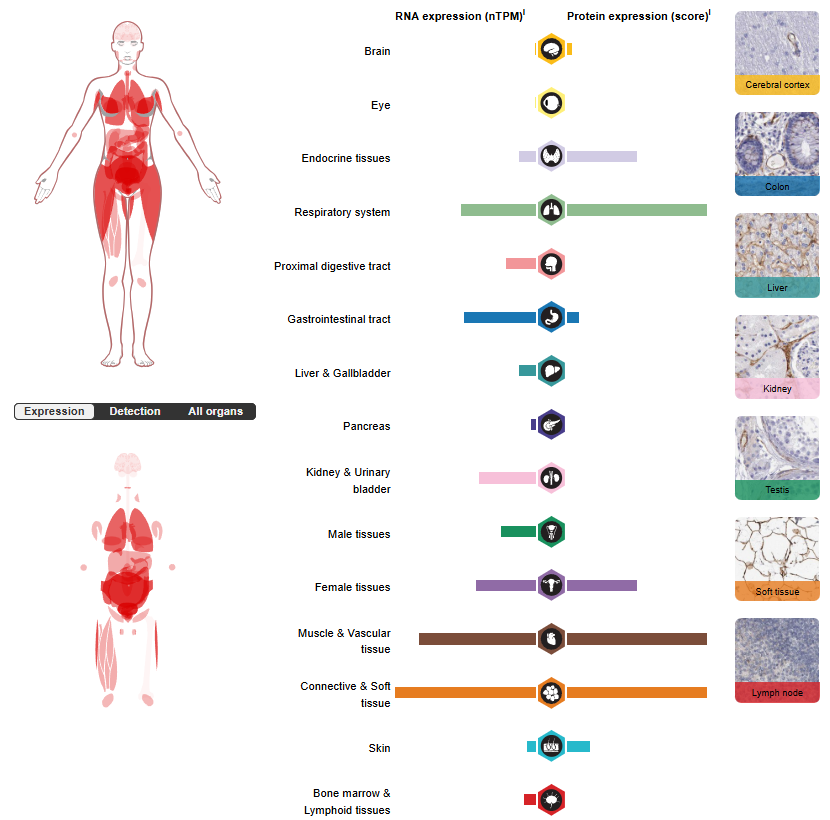
(Data source: uniprot)
Structure of VAP1 and its receptor
VAP-1 is a type II transmembrane glycoprotein, and its unique structure is the material basis for its dual function as an adhesion molecule and an oxidase.
The basic structure consists of a short intracellular tail region, a single transmembrane domain, and a large extracellular domain. This extracellular domain is highly glycosylated and contains the enzyme's active site.
Active site and catalytic mechanism: VAP-1 belongs to the copper-containing amine oxidase family. Its active site contains a copper ion (Cu²⁺) and a quinone cofactor formed by the oxidation of a specific tyrosine residue. This enzyme can catalyze the oxidative deamination of primary amines (such as methylamine) to produce the corresponding aldehyde, hydrogen peroxide (H₂O₂), and ammonia. This catalytic reaction is key to its signal transduction function.
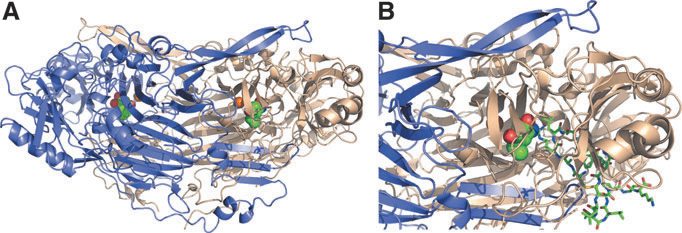
(Data source: Salmi M, et al. Antioxid Redox Signal. 2019)
The role of VAP1 in cardiovascular disease
VAP-1 is a multifunctional adhesion molecule that plays a crucial role in the pathogenesis of atherosclerotic cardiovascular disease (ASCVD). It exerts its effects by modulating inflammation, inducing vascular endothelial damage, regulating glucose and lipid metabolism, and altering plaque stability. Numerous studies have confirmed the close association between VAP-1 and the occurrence and prognosis of ASCVD.
Vascular inflammation induces VAP-1 translocation to the endothelial lumen surface, where it interacts with Siglec-9/10 (sialic acid-binding immunoglobulin-like lectin 9/10), mediating monocyte infiltration into atherosclerotic lesions. Simultaneously, enzymatic products of VAP-1, such as H2O2, may further increase VAP-1 expression on endothelial cells and subsequent monocyte migration. Early inflammatory lesions stimulate medial smooth muscle cells to transition from a quiescent "contractile" phenotype to an active "synthetic" state characterized by increased proliferation, migration, and collagen synthesis. VAP-1 on the smooth muscle cell membrane may inhibit this phenotypic transition through its enzymatic products, thereby limiting the growth of the fibrous cap composed of smooth muscle cells and collagen. Therefore, VAP-1 is a pathological factor in plaque instability, characterized by a thin fibrous cap covering a large lipid core rich in monocyte/macrophage-derived foam cells.

(Data source: Chen C, et al. Front Cardiovasc Med. 2025)
VAP1 targeted therapy
Timolumab (BTT1023) is a monoclonal antibody targeting VAP1, initially developed by Biotie Therapies (now Acorda) and its partners. It is currently in Phase 2 clinical trials for the treatment of primary sclerosing cholangitis. Timolumab inhibits VAP-1, which is believed to cause inflammation by promoting leukocyte aggregation and invasion of inflammatory sites. While conventional anti-inflammatory drugs inhibit the production or action of inflammatory substances, SI-3106 uniquely targets adhesion molecules that control leukocyte motility. It has been found to be safe and effective in treating psoriasis and rheumatoid arthritis (NCT00871598 and NCT00851240). A multicenter, open-label, single-arm study (NCT02239211) is underway to determine the safety, pharmacokinetics, and efficacy of BTT1023 treatment. Early clinical studies in psoriasis and rheumatoid arthritis have shown it to be well-tolerated and effective. Conversely, the phase II study in 19 patients with primary sclerosing cholangitis was discontinued after an interim analysis because the experiment did not meet the pre-defined efficacy requirements.

(Data source: Seikagaku official website)
