P-selectin glycoprotein ligand 1 (SELPLG), also known as CD162 and PSGL-1, is a cell adhesion molecule involved in cell rolling mechanisms and extravasation cascades, enabling immune cells to be recruited to sites of inflammation. PSGL-1 has multiple functions in lymphocytes, including inhibiting the proliferation of effector T cells, promoting T cell exhaustion, limiting their survival and regeneration, reducing the frequency of memory T cell generation, and promoting the immune regulation of Tregs. PSGL-1 and VISTA are a pair of immune checkpoints ; both PSGL-1 and VISTA exert inhibitory effects under acidic conditions.

(Data source: Peng M, et al. Biomark Res. 2024)
PSGL-1 expression distribution
PSGL-1 is widely expressed in hematopoietic cells, including bone marrow cells and lymphocytes, as well as platelets; neutrophils, dendritic cells, glial cells, NK cells, and T cells. In specific pathological conditions, such as anaplastic large cell lymphoma (ALCL), its expression can be abnormally elevated.

(Data source: uniprot)
Structure of PSGL-1 and its ligands
PSGL-1 is a 120 kDa type I transmembrane glycoprotein, typically existing as a 240 kDa homodimer. It is encoded by the SELPG gene located on chromosome 12q24.11. PSGL-1 interacts with a variety of ligands, including selectins P, E, and L, CCL19 and CCL21, Siglec-5, amidase LytA, and VISTA.
PSGL-1 is divided into three domains: extracellular domain (318 amino acids), transmembrane domain (24 amino acids), and cytoplasmic domain (70 amino acids).
Extracellular domain: N-terminal glycosylation is essential for binding P-selectin, E-selectin, and L-selectin. Furthermore, the extracellular domain of mature PSGL-1 is rich in threonine, proline, and serine, forming its tenosomal glycoprotein backbone.
Transmembrane domain: Contains cysteine residues that form disulfide bonds and promote dimerization.
Cytoplasmic domain: mainly interacts with ERM proteins, which support PSGL-1-induced signaling.
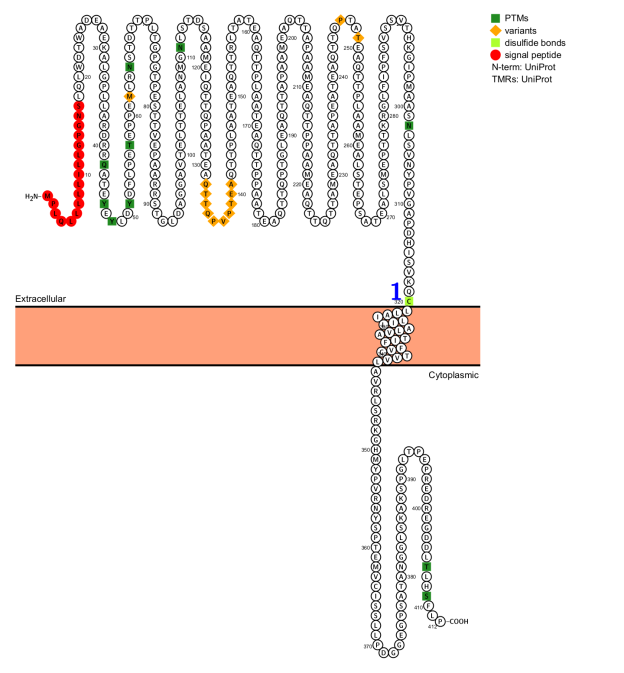
(Data source: uniprot)
PSGL- 1 Functions
PSGL-1 is an inhibitory checkpoint that promotes T cell exhaustion: when T cells are stimulated by tumor or viral antigens and bind to PSGL-1, TCR signaling is weakened through ERK and AKT dephosphorylation. Dysfunctional T cells exhibit reduced cytokine production, decreased survival rate, and upregulation of inhibitory receptors, which contribute to T cell exhaustion.
T cell subsets are regulated by PSGL-1: Naïve T cells can use PSGL-1 to enter and leave lymph nodes. In lymph nodes, PSGL-1 on naïve T cells binds to chemokines CCL-19 and -21 produced by stromal cells, which support their migration and survival. Naïve T cells transform into effector T cells, in which PSGL-1 plays a role, inhibiting effector cell proliferation and differentiation, and limiting the size of their response. Antigen persistence leads to PSGL-1 signaling, thereby reducing T cell survival, increasing inhibitory receptor expression, and inhibiting TCR signaling and cytokine production, thus promoting T cell exhaustion. After antigen clearance, PSGL-1 inhibits the formation of memory T cells and promotes the homing of memory T cells to lymphoid and non-lymphoid tissues.

(Data source: Tinoco R, et al. Trends Immunol. 2017)
Interaction with VISTA: In acidic microenvironments (such as tumor foci), PSGL-1 can interact with the checkpoint molecule VISTA, synergistically inhibiting T cell function . In myeloid cells, PSGL-1 expression on hard dura mater and macrophages has been shown to induce the formation of tolerant dura mater and M2. VISTA is not only expressed as a ligand on myeloid cells, regulating cytokine production, chemotaxis, and phagocytosis to help them differentiate into a tolerant phenotype, but also acts as a receptor on T cells, participating in T cell quiescence and inhibiting T cell activation. Furthermore, VISTA is highly expressed in MDSCs to promote immunosuppression.


(Data source: Peng M, et al. Biomark Res. 2024)
PSGL-1 targeted therapy
Torapsel is an Fc fusion protein developed by Pfizer that targets PSGL-1 for the treatment of cardiovascular, digestive, and genitourinary diseases. The highest stage of its development was Phase 2 clinical trials, which have since been terminated, and no new progress has been found.
VTX-0811 is a first-in-class monoclonal antibody being developed by Verseau that binds to a novel target and repolarizes macrophages, specifically targeting PSGL-1. Verseau discovered that regulation of specific epitopes on PSGL-1 can lead to repolarization of M2-to-M1 macrophages without affecting the normal function of PSGL-1. Upon binding to PSGL-1, VTX-0811 induces activation of the tumor microenvironment, thereby activating the primary recruitment of T cells and immune cells to form a coordinated immune attack against the tumor. In in vitro studies using patient-derived primary tumors, VTX-0811 demonstrated a stronger inflammatory response than existing immunotherapies in both PD-1-responsive and non-responsive tumors. In various syngeneic preclinical tumor models and a humanized mouse PDX melanoma model, VTX-0811 showed superior antitumor responses compared to PD-1 therapy.


(Data source: Verseau official website)
