Background
Epithelial cell adhesion molecule (EpCAM), a tumor antigen targeted by antibody-drug conjugates (ADCs), is highly expressed in many epithelial cancers. However, clinical advancement of EpCAM ADCs has been challenging, primarily due to their toxicity in tissues normally expressing it, such as the gastrointestinal tract. CLDN3 is co-expressed with EpCAM in upper gastrointestinal malignancies and is minimally expressed in normal tissues, making it an ideal target for the development of highly effective therapeutic ADCs.
On March 8, 2025, an article titled "Development of a bispecific antibody-drug conjugate targeting EpCAM and CLDN3 for the treatment of multiple solid tumors" was published in Exp Hematol Oncol. This article describes the development of a bispecific ADC (BsADC) targeting EpCAM and CLDN3, designed to avoid toxicity in normal tissues with high EpCAM expression. Parental monoclonal antibodies were screened for high binding and endocytosis activity against tumor cell lines. These were then modified into monovalent structures, and clones with reduced binding and endocytosis activity were selected. These clones were combined into bispecific antibodies (BsAbs), and molecules with restored binding and endocytosis activity were ultimately selected as lead molecules. Drutecan (Dxd) was conjugated to the BsAbs via a cleavable linker to generate BsADCs. High expression of EpCAM and CLDN3 effectively inhibited tumor cell growth, demonstrating their antitumor activity. Importantly, their binding to cells with high EpCAM expression but low CLDN3 expression was weak, suggesting minimal toxicity in normal tissues with high EpCAM expression. Furthermore, BsADCs exhibited favorable pharmacokinetics and low toxicity in mice. These findings suggest that BsADCs targeting EpCAM and CLDN3 may be promising for the treatment of various solid tumors.

EpCAM and CLDN3 are co-overexpressed in various tumors
EpCAM shows widespread and elevated expression in various cancers , particularly in colorectal malignancies, but it is also often moderately expressed in healthy tissues, primarily in the gastrointestinal tract, kidney, and pancreas, thus presenting the potential for toxicity in normal tissues.
Studies have found that the expression pattern of CLDN3 closely resembles that of EpCAM in 33 human cancer types . CLDN3 is highly expressed in tumor cells but not in normal tissues. CLDN3 is a promising tumor-associated antigen (TAA) that can be used as a target for the development of antibody-drug conjugates (BsADCs) that bind to EpCAM for the treatment of various solid tumors.

Selection and preparation process of BsADCs parent antibodies
The researchers selected clones that exhibited significantly reduced binding and internalization activity in the monovalent format, further minimizing the risk of toxicity in non-malignant tissues. Antibodies to gp120 were used as the nonbinding arm of the EpCAM x gp120 or gp120 x CLDN3 bsAbs with the EpCAM or CLDN3 monovalent arms. Knob-into-hole technology was used to avoid heavy chain mispairing, and crossmab technology was used to prevent light chain mispairing . Their binding and internalization activities were evaluated in ovarian cancer cells (OVCAR-3) and lung cancer cells (NCI-H1781). Most bsAbs restored binding and internalization activities compared to the parental monoclonal antibodies. Three bsAbs (BsAb1, EpCAM-AXCLDN3-C; BsAb2, EpCAM-BXCLDN3-C; and BsAb3, EpCAM-DXCLDN3-C) exhibited improved binding and internalization activities, likely due to synergistic effects between the two groups. The binding activity of all three bsAbs against OVCAR-3 tumor cells was dose-dependent.

EpCAMXCLDN3 BsADCs exhibit significant anti-tumor activity both in vitro and in vivo
EpCAMXCLDN3 BsADCs were generated by bioconjugating the Dxd cargo to three endogenous cysteine residues on the BsAb via a GGFG linker.
The cytotoxicity of BsADCs was tested in vitro . A dose-dependent antiproliferative response was observed in OVCAR-3 and NCI-H1781 cancer cells, which highly express EpCAM and CLDN3. The drug-conjugated BsADCs significantly enhanced their growth inhibitory activity against EpCAM- and CLDN3-positive cells and exhibited targeted, specific growth inhibition against these cells.
The in vivo antitumor activity of BsADCs was evaluated in a mouse xenograft model , and comparison of tumor growth on day 28 showed that BsADC treatment resulted in significant tumor regression compared with IgG-ADC treatment, with BsADC 3 showing the best antitumor effect.

BsADC 3 has low toxicity to normal tissues expressing EpCAM
The EpCAMxCLDN3 bispecific antibody-drug conjugate (BsADC) showed reduced binding and cytotoxic activity on cells with high EpCAM expression and low CLDN3 expression.
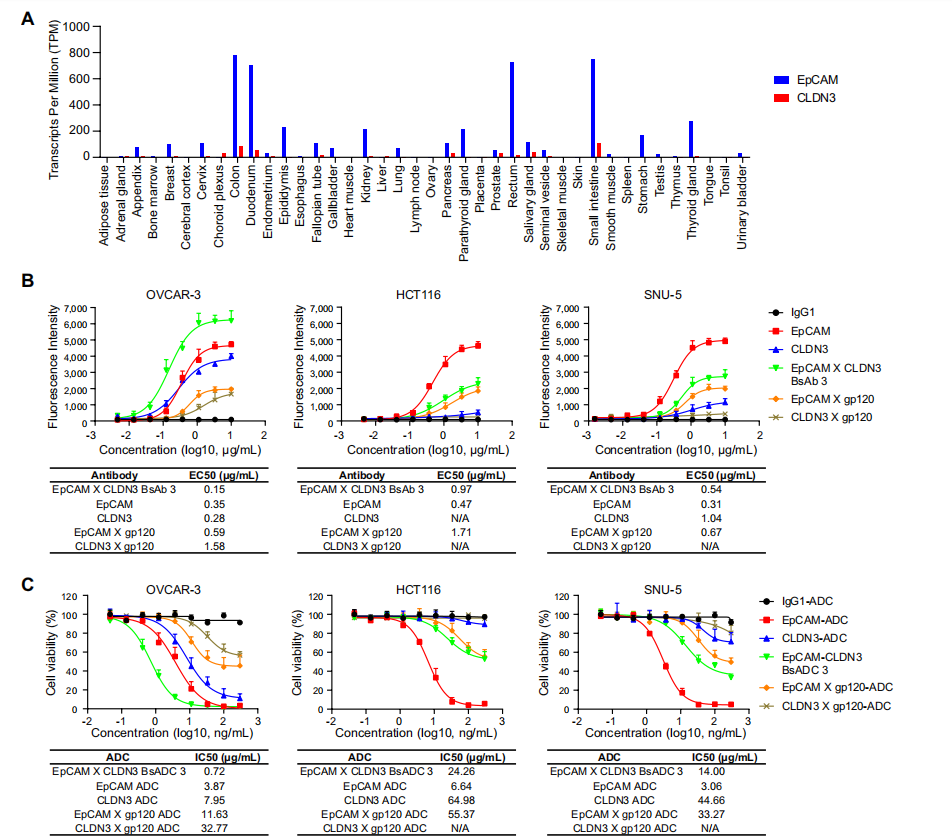
BsADC molecules have strong binding activity against tumor cells with high expression of EpCAM and CLDN3 and exhibit stronger ADCC, ADCP, and CDC effects on OVCAR-3 cells than their parental bivalent antibody. BsADC molecules bind weakly to HCT116 cells, which have high EpCAM expression but low CLDN3 expression, and their ADCC, ADCP, and CDC effects on HCT116 cells are also weaker than those of the parental bivalent antibody for EpCAM, potentially minimizing toxic side effects to normal tissues. BsAb3 exhibits high ADCC, ADCP, and CDC activity against OVCAR-3 cells but relatively low activity against HCT116 cells, further suggesting that BsAb3 may reduce off-target toxic effects on normal tissues while retaining cytotoxic effects on tumor cells.
BsADC 3 can maintain high cytotoxic activity against dual-high-expressing tumor cells while reducing the cytotoxic activity against cells with moderate EpCAM expression, thereby helping to expand the clinical therapeutic window.


BsADC 3 exhibits favorable pharmacokinetics and safety in mouse models
BsADC 3 has IgG1 kinetics and good in vivo stability , and EpCAM x CLDN3 BsADC 3 has good PK and safety, and has potential application value in the treatment of tumors expressing EpCAM and CLDN3.

Summarize
BsADCs hold tremendous potential as one of the most effective biotherapeutics. Numerous key factors must be considered in developing a well-designed bsADC, including target selection, format, binding affinity, and functional activity. Appropriate target selection fundamentally determines the mechanism of action of a bsADC and is crucial for its success. CLDN3 and EPCAM are highly expressed in various tumors, while CLDN3 is expressed at low levels in normal tissues, making it an attractive target for developing antibody therapeutics. EpCAM x CLDN3 BsADCs have demonstrated promising efficacy in treating various tumors in vitro and in vivo . BsADCs are designed to enhance the selectivity of payload delivery, enhance endocytosis to improve efficacy, minimize the risk of toxicity to non-malignant tissues, and overcome tumor cell escape mechanisms. BsADC-based therapies have the potential to revolutionize existing cancer treatment options and achieve significant progress in the fight against cancer.
