Epidermal growth factor receptor (EGFR), also known as HER1 and ERBB, is a receptor tyrosine kinase. EGFR is a cell surface protein that binds to epidermal growth factor, inducing receptor dimerization and tyrosine autophosphorylation, leading to cell proliferation. EGFR mutations or overexpression often contribute to tumors, such as non-small cell lung cancer (NSCLC).
Distribution of EGFR
EGFR is widely distributed on the cell surfaces of mammalian epithelial cells, fibroblasts, glial cells, keratinocytes, etc. The EGFR signaling pathway plays an important role in physiological processes such as cell growth, proliferation and differentiation.
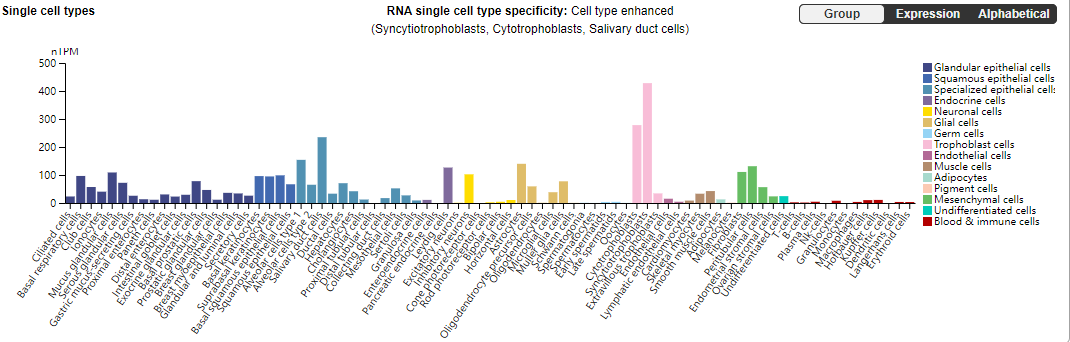
(Data source: uniprot)
Structure of EGFR
EGFR is a transmembrane glycoprotein with a molecular weight of approximately 170 kDa. EGFR consists of a cysteine-rich extracellular ligand-binding domain, a hydrophobic transmembrane domain, a cytoplasmic RTK domain, and a C-terminal domain. The extracellular regions L1 and L2 are the receptor ligand-binding sites, while CR1 and CR2 are rich in cysteine residues and are responsible for receptor dimerization. Its intracellular structure comprises a tyrosine kinase domain and a carboxy-terminal tail with multiple autophosphorylation sites. Small molecule EGFR tyrosine kinase inhibitors (TKIs) act within this intracellular domain. Multiple mutations within the tyrosine kinase domain are associated with both resistance and sensitivity to EGFR-TKIs.
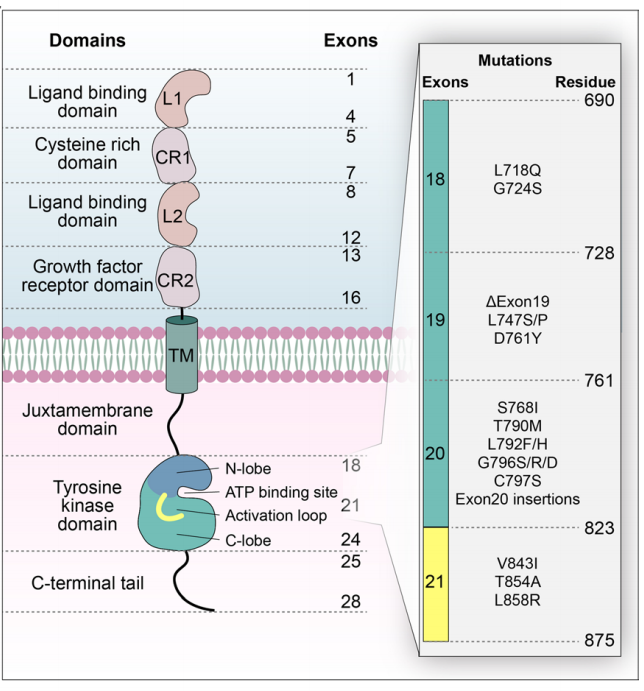
When EGFR is active, the key catalytic residue D855 in the tyrosine kinase domain is located in the ATP binding site, stabilizing the ATP loading complex (DFG-in) and the αC helix (αC-in). In the inactive state, EGFR forms a Src-like structure consisting of a closed A loop, αC-out, and DFG-in.
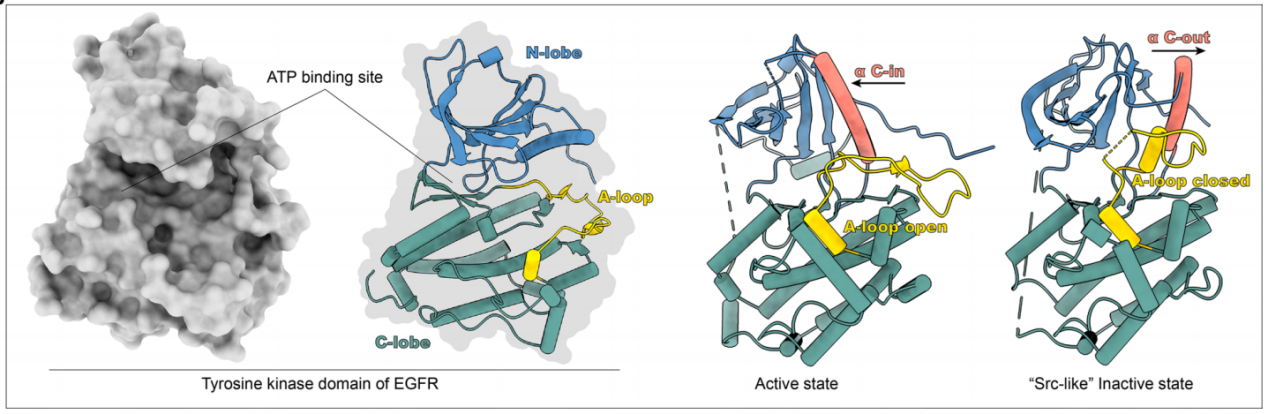
(Data source: Shi K, et al. J Hematol Oncol. 2022)
EGFR signaling pathway regulation
Binding of EGFR to specific ligands (such as EGF and TGF-α) leads to homo- or heterodimerization of the receptor, which in turn causes conformational changes in the intracellular kinase domain, leading to receptor phosphorylation and activation. The signaling axes RAS/RAF-MAPK and PI3K-AKT-mTOR in turn activate various downstream signaling pathways, resulting in enhanced cell proliferation and survival.

(Data source: Shi K, et al. J Hematol Oncol. 2022)
Mechanisms of resistance to EGFR drugs
The drug resistance mechanisms of EGFR mainly include secondary mutations of the EGFR gene (T790M mutation, C797S mutation), other EGFR mutations (such as L718Q, L796S and L792H mutations and exon 20 insertion), MET amplification, phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit α (PIK3CA) mutation, HER2 amplification, oncogene fusion and changes in cell cycle-related genes.
Both amplification and mutation of receptor tyrosine kinases (RTKs) can induce downstream survival signaling pathways. Furthermore, direct overexpression and/or mutation of downstream pathway components can promote acquired drug resistance by promoting cancer cell survival.
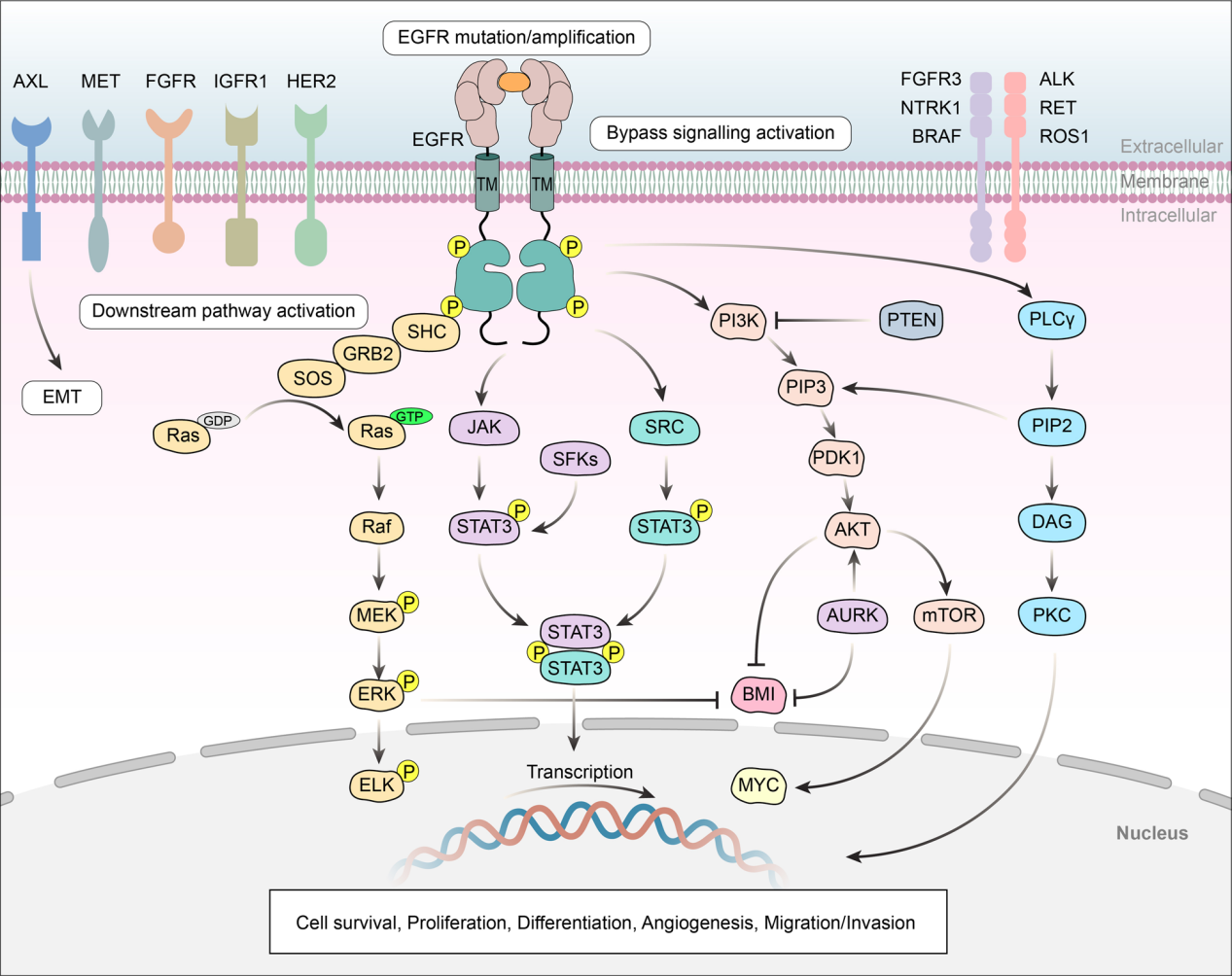
(Data source: Shi K, et al. J Hematol Oncol. 2022)
EGFR-targeted therapy
Currently, there are two main approaches to targeting EGFR: small molecule kinase inhibitors targeting the intracellular domain and monoclonal antibodies targeting the EGFR extracellular domain or antagonizing ligand-receptor interactions. However, acquired drug resistance hinders the achievement of therapeutic results. Recent advances in anti-ErbB approaches, such as bispecific antibodies (BsAbs) and antibody-drug conjugates (ADCs), aim to overcome this resistance.
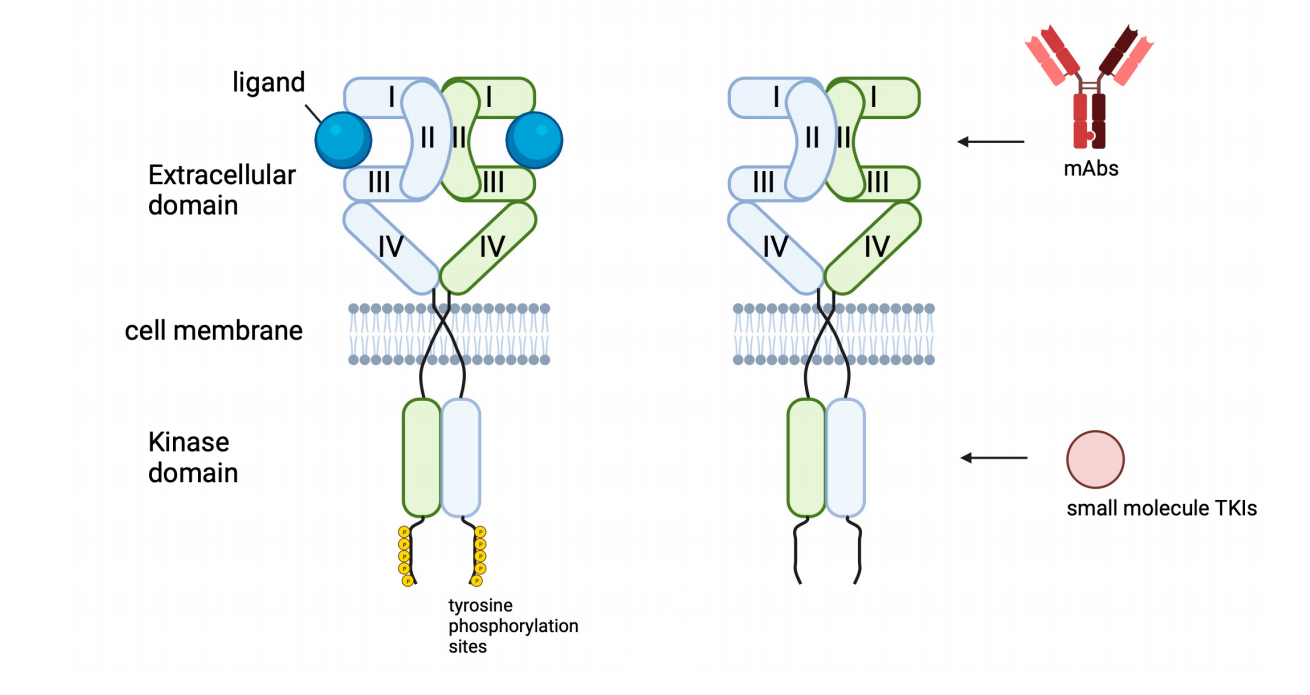
(Data source: Shaban N, et al. Cells. 2023.)
Small molecule kinase inhibitors of EGFR
Small molecule drugs competitively inhibit the ATP binding site of EGFR, preventing its kinase activity and thus inhibiting the proliferation of tumor cells. Based on the development time and mechanism of action, they can be divided into three generations:
First-generation TKIs: reversible, non-selective inhibitors; such as gefitinib and erlotinib.
Second-generation TKIs: irreversible, non-selective inhibitors; such as afatinib, dacomitinib, etc.
Third-generation TKIs (targeting T790M mutation): irreversible, selective inhibitors; such as osimertinib, alectinib, etc.

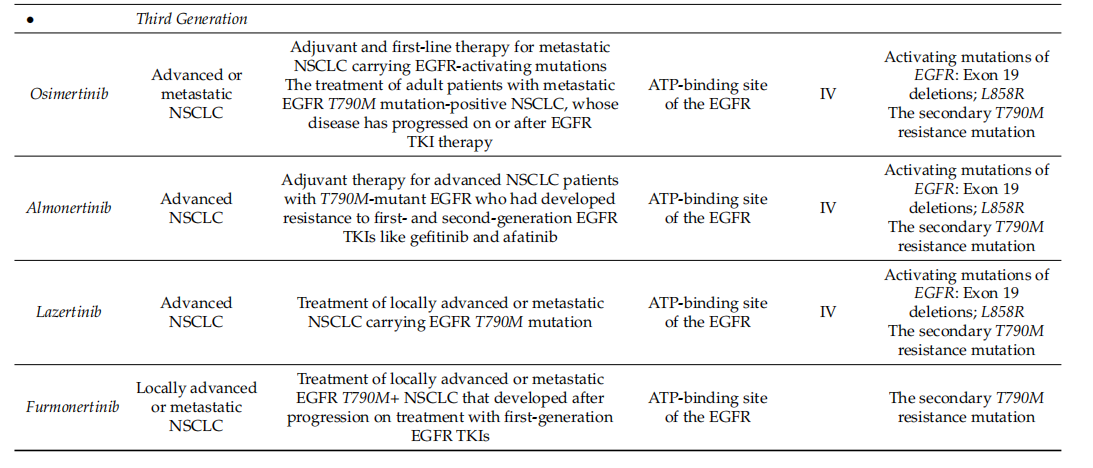
(Data source: Shaban N, et al. Cells. 2023)
EGFR monoclonal antibodies
Anti-EGFR monoclonal antibodies bind to the extracellular domain of the EGFR protein, inhibiting signal transduction, thereby preventing cell proliferation and survival, ultimately leading to apoptosis. Approved monoclonal antibodies targeting EGFR include cetuximab, Enrituximab (cetuximab beta injection), nimotuzumab, netotuzumab, and panitumumab. In addition, many other antibodies are currently under clinical research.
Enlito is an EGFR-targeted monoclonal antibody approved for marketing by the China National Medical Products Administration (NMPA) in June 2024. It was jointly developed by oncology drug company Simcere and Maibo Pharmaceuticals. This drug fills a nearly 20-year gap in China's EGFR-targeted antibody pipeline for colorectal cancer, which has been dominated by imported drugs.
Becotatug is a monoclonal antibody targeting EGFR developed by Jinmant Biopharma, a subsidiary of Shijiazhuang Pharmaceutical Group. It is used to treat EGFR-positive non-squamous non-small cell lung cancer, EGFR-mutated non-small cell lung cancer, EGFR exon 20 insertion mutation non-small cell lung cancer and other diseases. It is currently in the Phase 3 clinical research stage.
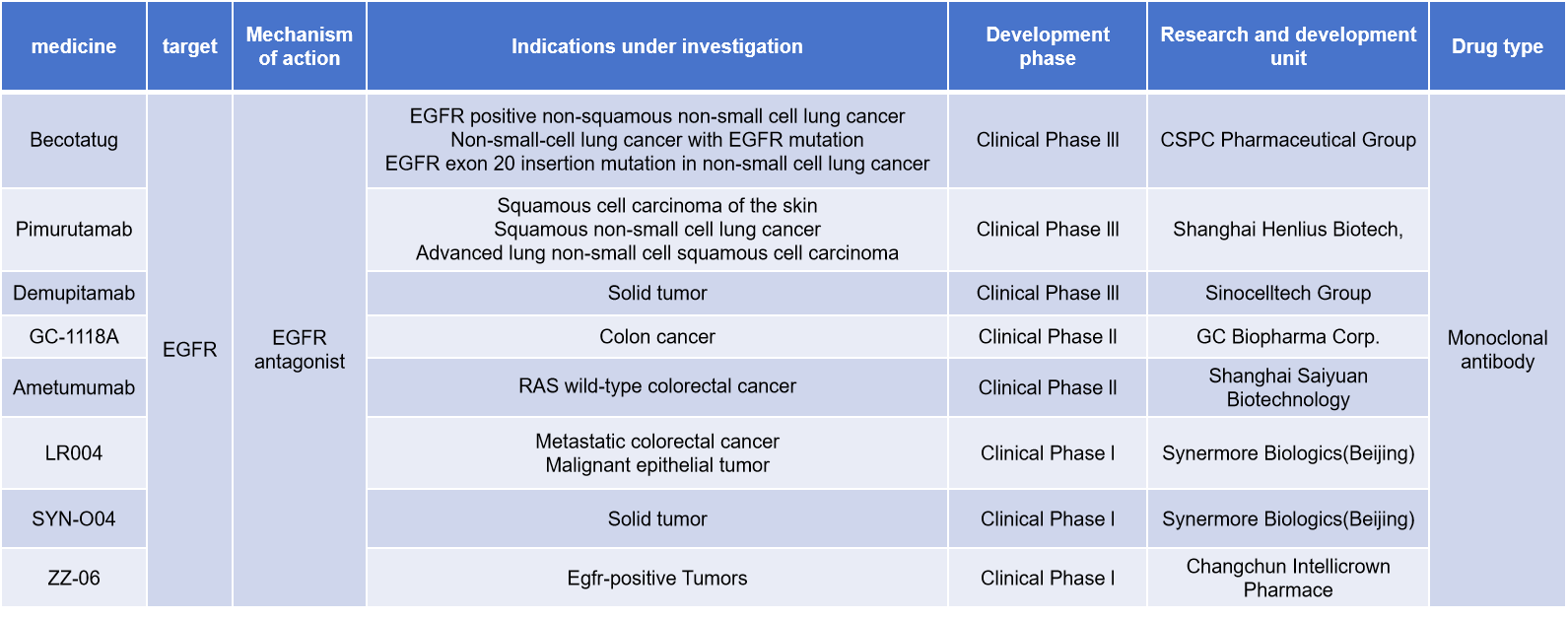
(Data source: New Drug Intelligence Database)
EGFR/c-MET dual-target drug development
EGFR and c-MET plays a complex and important role in tumor biology. Due to abnormal activation of c-MET, amplification or overexpression of c-MET can bypass EGFR activation of downstream signaling pathways, such as PI3K/Akt and MAPK, thereby promoting tumor cell survival and proliferation. Bispecific antibodies targeting EGFR and c-MET can simultaneously block EGFR- and c-MET-mediated signaling, thereby inhibiting tumor cell growth and survival. The development of such bispecific antibodies provides a new therapeutic strategy for overcoming EGFR-TKI resistance.
Amivantamab, a bispecific antibody targeting EGFR and c-MET, was approved by the FDA in 2021. Developed by Johnson & Johnson, it is the world's first targeted therapy approved for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer harboring epidermal growth factor receptor exon 20 insertion mutations. Studies have shown that the combination of amivantamab and lazertinib has shown significant therapeutic efficacy in the treatment of patients with advanced non-small cell lung cancer (NSCLC) harboring EGFR mutations, extending patients' median progression-free survival (PFS).

(Data source: Felip E, et al. Ann Oncol. 2024)
There are currently many EGFR/c-MET dual-target drugs under development, including 4 bispecific antibodies, 1 triple antibody, and 2 ADC drugs.
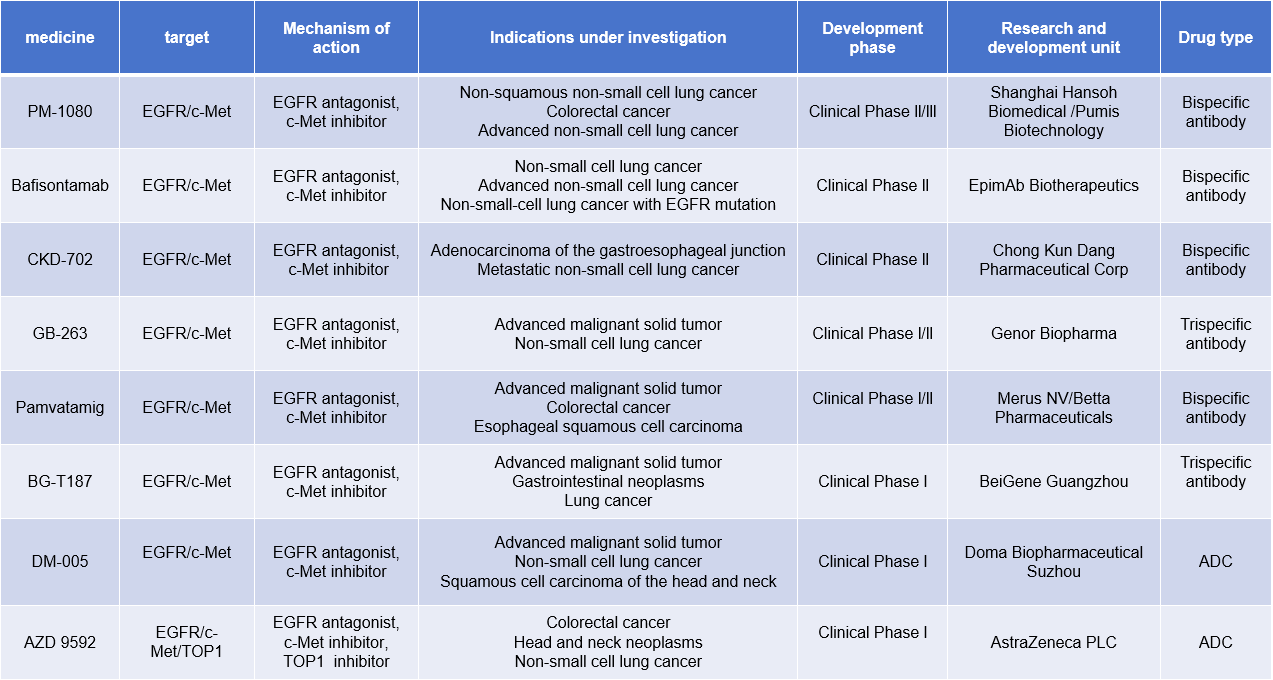
(Data source: New Drug Intelligence Database)
PM-1080 is a bispecific antibody targeting EGFR/c-MET developed by Biotheus Inc. It inhibits tumor growth and survival by specifically targeting the tumor antigens EGFR and c-Met. It is used to treat non-squamous non-small cell lung cancer, colorectal cancer, and advanced non-small cell lung cancer.
BG-T187, a trispecific antibody targeting EGFR/c-MET developed by BeOne Medicines, is in Phase I clinical trials for the treatment of advanced malignant solid tumors, gastrointestinal tumors, lung cancer and other diseases.
DM-005 is a fully human EGFR/c-MET bispecific antibody-drug conjugate (ADC) developed by Biopharma for the treatment of advanced malignant solid tumors, non-small cell lung cancer, head and neck squamous cell carcinoma, and other diseases. It is in Phase 1 clinical trials. DM-005's co-light chain bispecific antibody structure, developed using Biocytogen's RenLite platform, is structurally stable and highly pure. The linker-payload used in DM-005 is BLD1102 (a topoisomerase inhibitor). Compared to its parent monoclonal antibody, DM-005 exhibits stronger binding and internalization abilities against the double-positive tumor cell line NCI-H1975.
