Granulocyte macrophage stimulating factor (CSF2), also known as GM-CSF, is a cytokine that drives the activation of granulocytes, including neutrophils, monocytes, macrophages, and dendritic cells, in response to stress, infection, and cancer. GM-CSF plays an important role in tumor immunoregulation by regulating the function of innate immune cells and acting as a bridge to activate adaptive immune responses.
Expression distribution of CSF2
GM-CSF is produced by lymphocytes, macrophages, fibroblasts, endothelial cells, chondrocytes, and tumor cells in response to immunogenic stimuli.

(Data source: Uniprot)
The structure of CSF2 and its receptor
CSF2 is a secreted protein with a molecular weight of 23 kDa. The gene encoding it is located at 5q22-31 and has multiple α-helical and β-pleated structures. The GM-CSF receptor is composed of an α-chain and a signaling β-chain subunit. The β-chain is shared with the IL-3 and IL-5 receptors. The three-dimensional structure of the GM-CSF receptor is a hexameric complex composed of two GM-CSF molecules, two GMR α chains, and two βc chains. This structure enables GM-CSF to bind simultaneously to both receptor subunits, forming a high-affinity complex.

(Data source: Hercus TR, et al. Blood. 2009)
CSF2 signaling pathway and regulation:
After GM-CSF binds to the α chain of the GM-CSF receptor, it associates with the β chain to form a multimer. Receptor polymerization leads to the activation of JAK2, which phosphorylates tyrosine residues on the β chain. Phosphorylated tyrosines recruit STAT-5, which contains the SH2 domain. JAK2 activates STAT-5, thereby activating the JAK-STAT pathway. Furthermore, JAK2 may lead to the activation of PI3K, thereby initiating the PI3K-AKT pathway. Phosphorylated tyrosines on the GM-CSF receptor β chain recruit the adaptor protein SHC, which activates RAS and initiates the MAPK signaling pathway, inducing nuclear signaling. The JAK2-STAT-5 pathway primarily controls cell differentiation and inflammatory signaling, while PI3K signaling promotes cell proliferation and survival, and the MAPK pathway is involved in cell growth, proliferation, and differentiation.

(Data source: Kumar A, et al. Front Immunol. 2022)
The role of CSF2 in cancer
CSF2 has a double-edged sword effect in cancer, acting both to promote and suppress tumor growth.
Anti-tumor effects of CSF2
The immunostimulatory effects of GM-CSF contribute to its anticancer function: for example, GM-CSF restores neutrophil-driven immune responses in cancer, GM-CSF promotes antitumor immune responses by activating monocytes/macrophages and enhancing DC differentiation, and promotes anticancer T cell responses.
GM-CSF directly inhibits tumor cell growth: GM-CSF inhibits the proliferation of malignant cells by inducing arrest in the G0/G1 phase of the cell cycle and enhancing their differentiation. GM-CSF also inhibits tumor growth by inhibiting angiogenesis.
Tumor-promoting effects of CSF2
GM-CSF's immunomodulatory effects contribute to its tumor-promoting function. CSF2 can promote the generation of immunosuppressive cells (such as myeloid-derived suppressor cells, MDSCs), which can inhibit anti-tumor immune responses through multiple mechanisms. They also promote epithelial to mesenchymal transition (EMT), angiogenesis, and the expression of immune checkpoint molecules, thereby promoting cancer development.

(Data source: Kumar A, et al. Front Immunol. 2022)
CSF2-targeted therapy
Many GM-CSF-targeted drugs are under development, and their indications cover a variety of cancers, such as lymphoma, leukemia, nasopharyngeal tumors and other diseases.
Lenzilumab, a CSF2-targeting monoclonal antibody developed by Humanigen for the treatment of non-Hodgkin's lymphoma, acute graft-versus-host disease, and chronic myelomonocytic leukemia, was acquired by Taran Therapeutics in September 2024, following the sale of its biopharmaceutical business, including lenzilumab, ifabotuzumab, and HGEN005, to Taran Therapeutics for $20 million. The ACTRN12621000223831 PREACH-M study is a Phase 2 trial investigating the efficacy of lenzilumab and high-dose ascorbic acid combined with azacitidine, based on molecular profiling in participants with chronic myelomonocytic leukemia.
In October 2022, otilimab, a monoclonal antibody targeting CSF2, failed the Phase III ContRAst-3 study in moderate-to-severe rheumatoid arthritis (RA). Although the ContRAst-1 and ContRAst-2 trials met their primary endpoints, the demonstrated efficacy was unlikely to change the treatment of this difficult-to-treat patient population. Limited efficacy did not support an appropriate benefit/risk profile for otilimab as a potential treatment for RA. Consequently, GSK decided not to submit its application to regulatory authorities.
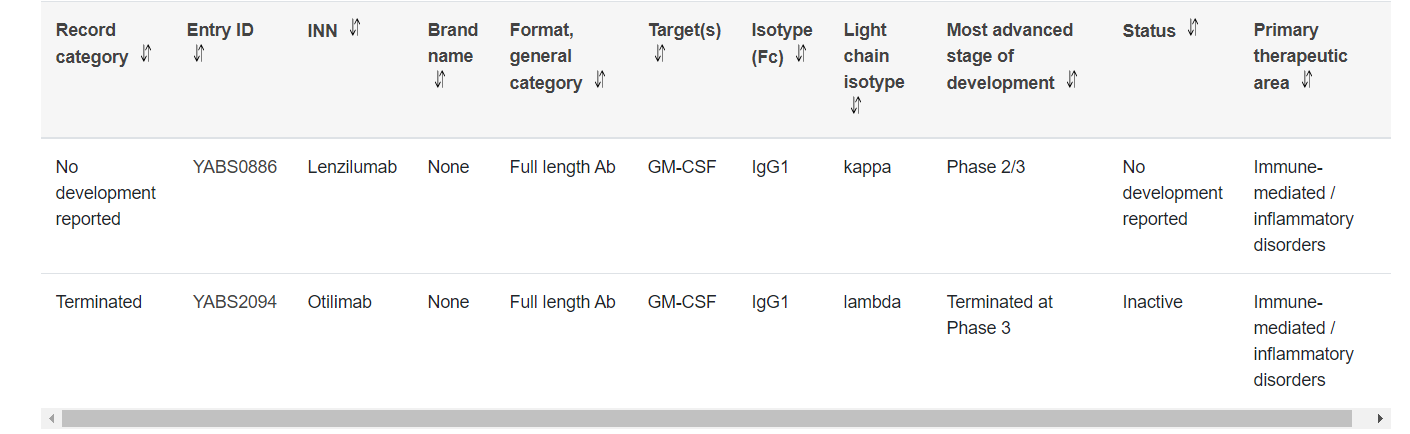
(Data source: YAbS database)
