V-type immunoglobulin domain-containing inhibitor of T-cell activation (VISTA), also known as VISR, is a member of the B7 family. It is an immunomodulatory receptor that inhibits T cell responses and is a novel negative checkpoint receptor. It is involved in maintaining immune tolerance, plays a role in tumor immune escape, and plays a role in autoimmune diseases. It is a potential target for combination cancer immunotherapy.
VISTA expression
At steady state, VISTA is expressed in various human tissues, particularly in the hematopoietic compartment and tissues containing infiltrating leukocytes, such as the bone marrow (BM), thymus, spleen, and lymph nodes.
In terms of hematopoietic tissues, peripheral blood mononuclear cells (PBMCs), mainly of the myeloid lineage, including monocytes, myeloid dendritic cells (DCs), macrophages, neutrophils and basophils, have the highest expression levels of VISTA.


(Data source: Uniprot)
Structure of VISTA
VISTA is a type I transmembrane protein (55-65 kDa) encoded by the VSIR gene, located at 10q22.1 within an intron of the CDH23 gene. The VISTA protein consists of a highly glycosylated extracellular Ig-V domain, a 33-aa stalk region, a transmembrane domain, and a cytoplasmic domain. The VISTA extracellular domain (ECD) adopts a canonical β-sandwich structure, with the H-, A-, G-, F-, C-, and C′ β-strands represented on the front side and the A'-, B-, E-, D-, and C′′ β-strands on the back side. The VISTA ECD, primarily the CC′ loop, contains a large number of positively charged histidine residues. These residues are responsible for forming a pH-dependent binding site, thereby enhancing VISTA binding to its ligand in acidic environments such as the tumor microenvironment.
VISTA contains three C-terminal Src homology domain 3 (SH3) binding motifs (PxxP). Human VISTA contains a Src homology domain 2 (SH2) binding motif (YxxQ), as well as multiple casein kinase 2 and phosphokinase C phosphorylation sites in the cytoplasmic domain. This enables VISTA to alter several cellular functions and act as both a receptor and a ligand . The cytoplasmic region of VISTA is crucial for its intracellular function, similar to members of the CD28 family, such as PD-1 and CTLA-4.
Human VISTA has two confirmed immunosuppressive ligands: P-selectin glycoprotein ligand-1 (PSGL-1) and VSIG3, containing three V-group and immunoglobulin domains. VISTA interacts with VSIG3 at physiological pH, but at acidic pH, VISTA-expressing cells can bind to PSGL-1 on T cells. Both interactions lead to inhibition of T cell function.

(Data source: Yuan L, Tatineni J, Mahoney KM, Freeman GJ. Trends Immunol. 2021)
The role of VISTA in tumor immunity
VISTA has been identified as a key mediator of cancer immune tolerance through its mechanism of suppressing the activation of tumor-reactive T cells and myeloid cells, and enhancing the presence and function of tolerogenic immune populations in the TME.
VISTA can directly inhibit T cell anti-tumor responses. In naive CD4+ T cells, VISTA may function as a receptor that maintains a quiescent or tolerant phenotype. Furthermore, VISTA may act as a ligand expressed by tumor cells, signaling on T cells through putative ligands and inhibiting T cell receptor-mediated activation. VISTA can inhibit the inflammatory activation of myeloid immune cells. Myeloid cells recognize and respond to damage-associated molecular patterns released by cancer cells through Toll-like receptors (TLRs), thereby recruiting and activating adaptive immune responses. VISTA may inhibit downstream signaling of TLRs, thereby suppressing TLR-mediated proinflammatory cytokine production and preventing the activation of adaptive anti-tumor immunity. VISTA can increase the population of immunosuppressive cells in the TME, thereby impairing anti-tumor immunity. VISTA expression by myeloid-derived suppressor cells enhances their ability to suppress T cell activity. Furthermore, VISTA signaling in naive CD4+ T cells may stabilize FOXP3 expression and contribute to the maintenance of regulatory T cells in the TME.

(Data source: Zhang RJ, Kim TK. Exp Mol Med. 2024)
In PD-L1+ tumors, VISTA may promote primary or acquired resistance to anti-PD therapy by increasing immunosuppressive cell populations, including immunosuppressive myeloid immune cells. Tumor cells may also suppress the antitumor activity of T cells by expressing or upregulating VISTA through a putative receptor. In PD-L1- tumors, anti-PD therapy is ineffective due to the lack of a target. T cell suppression may be mediated by other immune checkpoint pathways, including those using VISTA as a receptor or ligand. VISTA may increase immunosuppressive cell populations in the TME or directly inhibit T cell activity. Anti-VISTA blockade, particularly as part of combination therapy, alters the phenotype of myeloid immune cells within the TME, making them more proinflammatory and potentially enhancing T cell activation, ultimately leading to improved antitumor immune responses.

(Data source: Zhang RJ, Kim TK. Exp Mol Med. 2024)
VISTA targeted therapy
VISTA uses distinct signaling pathways from CTLA-4 and PD-1/PD-L1, synergizes with anti-CTLA-4 and -PD-1, and contributes to resistance to anti-CTLA-4/PD-1 therapy. Therefore, targeting VISTA within the TME could inhibit tumor promotion and induce anti-tumor responses. Multiple recent preclinical and clinical studies have demonstrated that VISTA has significant therapeutic potential, both as an agonist and an antagonist.
CA-170 is a small molecule inhibitor that targets the VISTA (H chain) and PD-L1/L2 pathways without disrupting the PD-1/PD-L1 interaction. A Phase I clinical trial (NCT02812875) demonstrated its safety and efficacy in the treatment of solid tumors and lymphomas. Patients experienced increased activation of CD4+ and CD8+ T cells in their peripheral blood. In 2020, Aurigene Oncology of India conducted a Phase IIb/III, randomized, double-blind, placebo-controlled, multicenter study of CA-170 in combination with chemotherapy and radiotherapy for the treatment of unresectable Stage III or IVa non-squamous NSCLC (ASIAD-2).
CI-8993 is a monoclonal antibody inhibitor targeting VISTA, and Curis is currently studying CI-8993 (Onvatilimab) in a Phase I clinical trial for the treatment of relapsed/refractory solid tumors (NCT04475523). Notably, even at subtherapeutic doses, CI-8993 triggers significant cytokine release, leading to neurotoxicity, likely due to its depletion of the IgG1 cytoskeleton.
HMBD-002 is an IgG4 anti-VISTA monoclonal antibody developed by Hummingbird Biosciences that primarily interacts with the CC ' loop of VISTA, where VISTA interacts with VSG -3 and LRIG1. It neutralizes VISTA function without depleting VISTA+ cells , through an FC -independent mechanism of action. In several humanized and syngeneic mouse models of breast, colorectal, and lung cancer, HMBD-002 demonstrated therapeutic efficacy, inhibiting tumor growth without significant toxicity. A preclinical study demonstrated that HMBD-002 exhibited excellent efficacy when combined with pembrolizumab (anti- PD - L1 ), particularly in tumors with high MDSC infiltration. The drug is currently in a Phase 1/2 clinical trial (NCT05082610) as a single agent and in combination with pembrolizumab for the treatment of advanced solid tumors expressing VISTA.
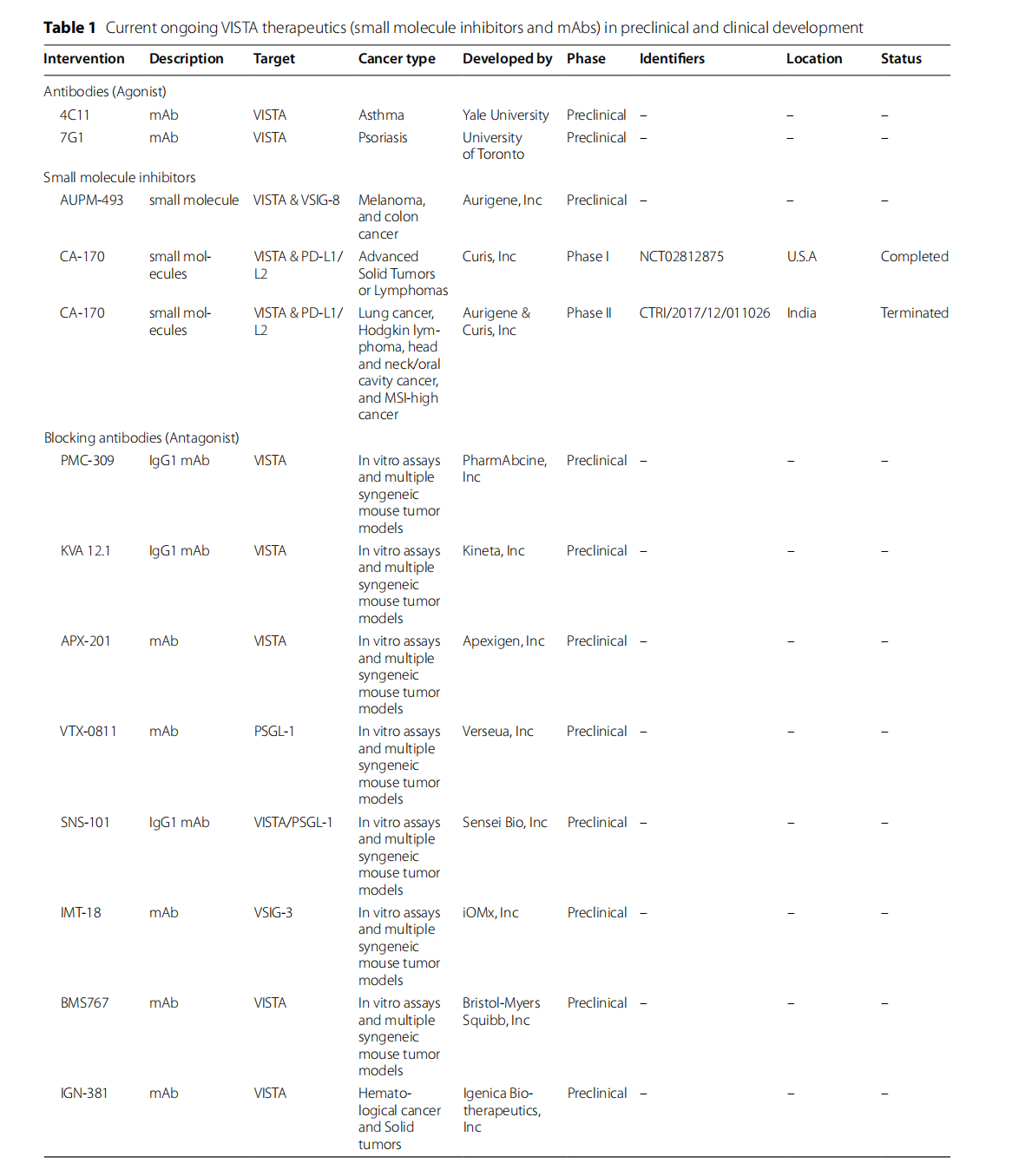

(Data source: Shekari N, et al. Cancer Cell Int. 2023)
