T cell-specific surface glycoprotein CD28, is a founding member of the immunoglobulin (Ig) costimulatory receptor family. It plays a crucial role in T-cell activation, proliferation, survival, and maintenance of immune homeostasis . In CD4+ and CD8+ T-cell subsets, stimulating binding of its cognate ligands, CD80 or CD86, increases the proliferation and expression of various cytokines, particularly IL-2 production. The CD28 family of receptors includes CD28, ICOS, CTLA-4, PD-1, and BTLA molecules.
Expression distribution of CD28
CD28 is mainly expressed in T cells, and is also expressed in Hofbauer cells, plasma cells, NK cells, granulocytes, monocytes, macrophages, Kupffer cells, dendritic cells, Langerhans cells, and erythroid cells.
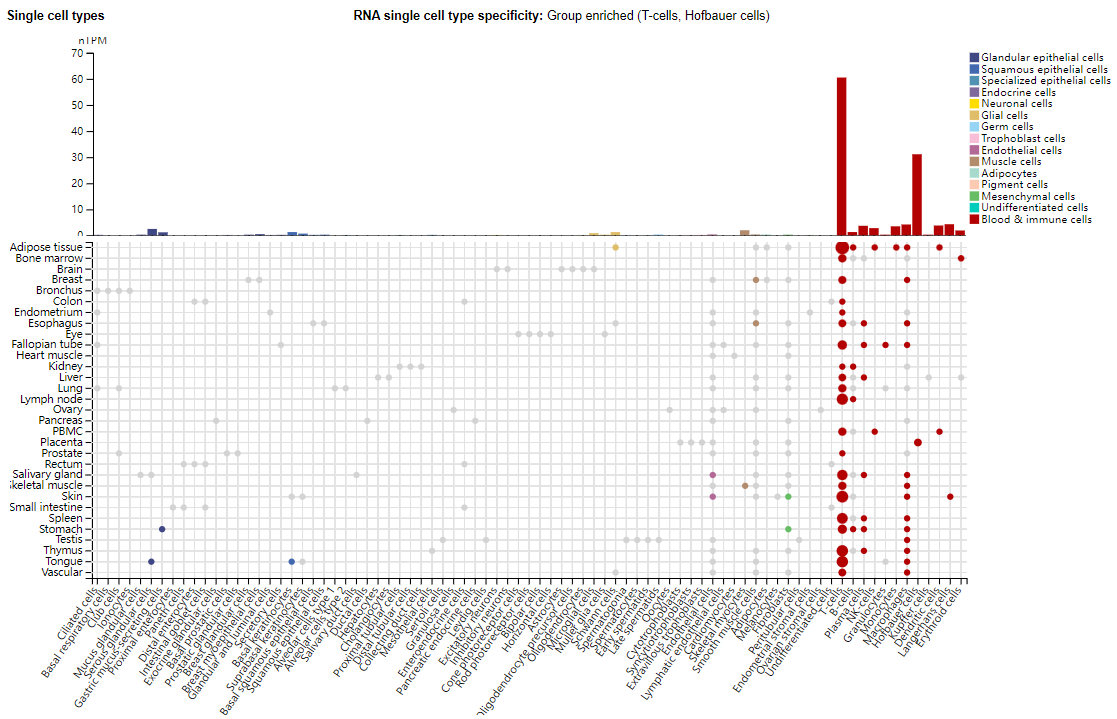
(Data source: Uniprot)
The structure of CD28 and its ligands
CD28 is a 220-amino acid type I transmembrane protein expressed on the cell surface as a glycosylated, disulfide-linked 44 kDa homodimer. Each subunit contains an extracellular domain, a transmembrane domain, and an intracellular domain.

(Data source: uniprot)
The major ligands for CD28 are CD80 and CD86 of the B7 family, which are commonly expressed on antigen-presenting cells (APCs) such as dendritic cells and activated B cells.
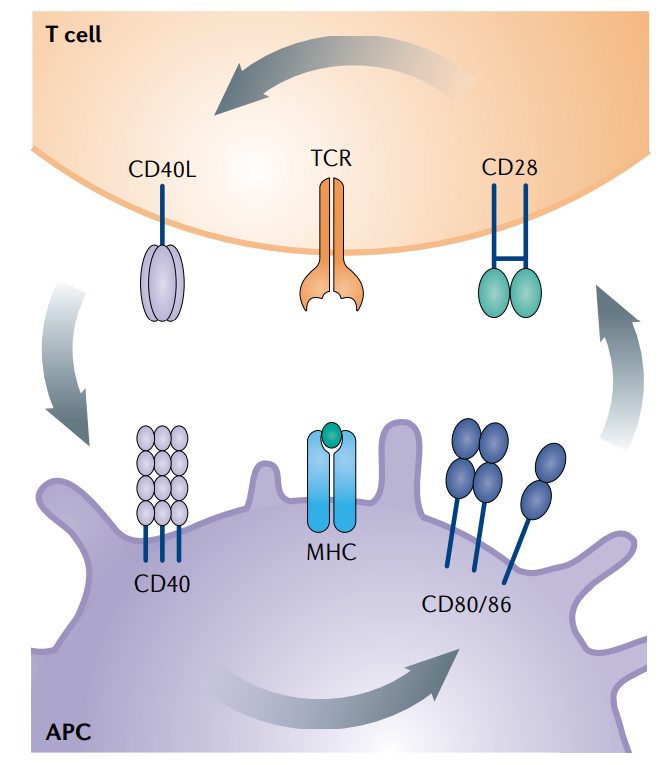
(Data source: Edner NM, et al. Nat Rev Drug Discov. 2020)
CD28 signaling pathway and regulation:
After the T cell receptor (TCR) recognizes an antigen, CD28 binds to CD80 or CD86, providing the " second signal " required for T cell activation . The intracellular domain of CD28 contains multiple signaling motifs, including PYAP, YMNM, and PRRP. These motifs play an important role in signal transduction.

After CD28 binds to CD80 or CD86, LCK (lymphocyte-specific protein tyrosine kinase) is recruited to the intracellular domain of CD28 and phosphorylates the tyrosine residues of CD28. Phosphorylated CD28 can recruit multiple signaling molecules, such as PI3K (phosphatidylinositol 3-kinase), GRB2 (growth factor receptor-binding protein 2), and ITK (T cell-specific tyrosine kinase).
These signaling molecules activate downstream signaling pathways, including transcription factors such as NF-κB , AP1 , NFAT , and mTOR . These transcriptional and translational effects collectively regulate T cell processes, including survival, proliferation, and cytokine production (including IL-2 and IFNγ).

(Data source: Lotze MT, Olejniczak SH, Skokos D. Nat Rev Immunol. 2024)
The role of CD28 in tumors
Tumor cells may inhibit T cell activation and proliferation by downregulating the expression of CD80 and CD86, reducing interactions with CD28. CD28 downregulation is a hallmark of CD8+ T cell senescence and aging in cancer patients. Indeed, an increase in the population of immunosuppressive CD8+CD28-senescent T cells has been observed in a variety of solid tumors and hematologic malignancies. Therefore, tumor-induced CD28 downregulation is associated with severe T cell senescence, which may be another mechanism by which tumor cells become resistant to immune surveillance and immunotherapy, and is associated with poor clinical outcomes in cancer . Sustained CD28 co-stimulation is required for the maintenance and expansion of TCF1+PD1+CD8+ T cells.

(Data source: Huff WX, et al. Int J Mol Sci. 2019)
CD28 -targeted therapy
CD28 plays an important role in cancer targeted immunotherapy. Therapeutic methods targeting CD28 mainly include bispecific antibodies, trispecific antibodies or multispecific antibodies targeting CD28.
Checkpoint inhibitors, such as antibodies against PD -1, PD-L1, and CTLA4, remove the “brakes” that prevent T cells from attacking cancer cells. However, if tumor cells do not express MHC The anti-tumor efficacy of checkpoint inhibitors may be limited if tumor-mutated peptides are presented to T cells via class I molecules (via endogenous signal 1 through the TCR-CD3 complex) and/or signal 2 (via binding to the CD28 receptor). Researchers are exploring combination therapy strategies, such as combining checkpoint inhibitors with CD28, tumor vaccines, and cytokine therapy, to enhance T cell activation signals and function, overcome tumor immune escape, and improve the efficacy of checkpoint inhibitors.
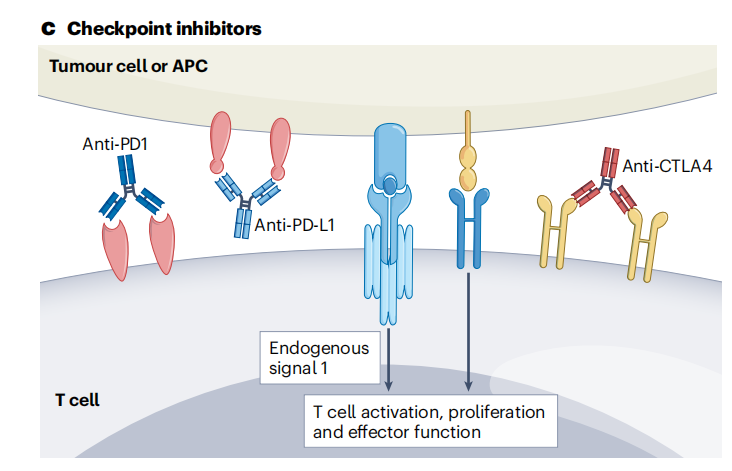
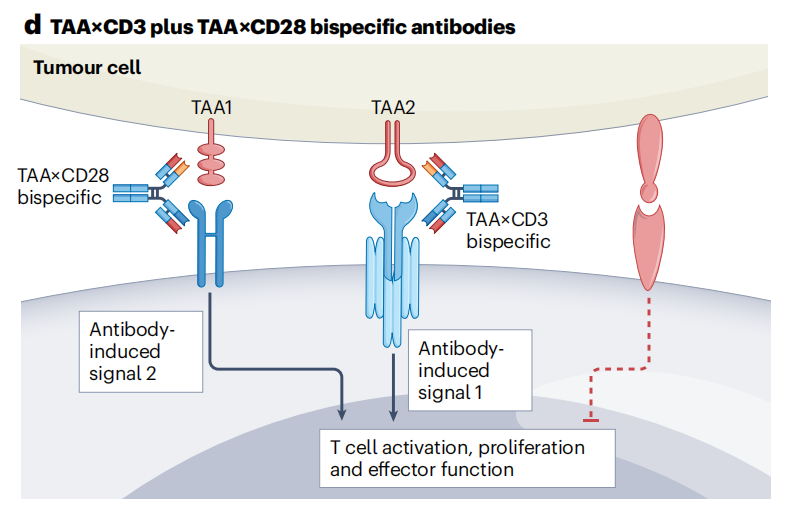
Bispecific antibodies
The combination of tumor-associated antigen 1 (TAA1) × CD28 and TAA2 × CD3 bispecific antibodies induces a nascent anti-tumor response by providing signal 1 and signal 2 to T cells when tumor cells express two tumor-associated antigens (TAAs), thereby enhancing target cell killing.
JNJ 87189401 is a bispecific antibody targeting PSMA/CD28, developed by Johnson & Johnson for the treatment of advanced prostate cancer and is in Phase 1 clinical research.
Bispecific antibodies combined with immune checkpoint therapy
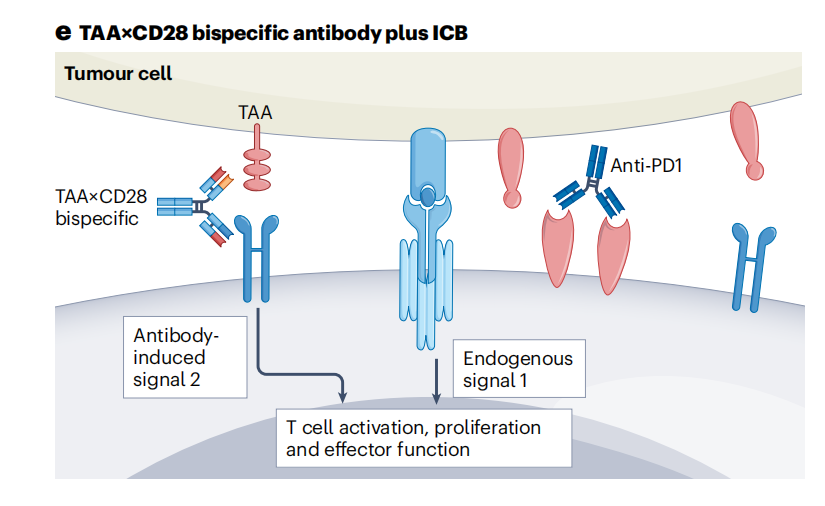
The efficacy of TAA × CD28 bispecific antibodies combined with immune checkpoint blockade (ICB) depends on pre-existing antitumor immunity. TAA × CD28 bispecific antibodies activate T cells when tumor cells present tumor-mutated peptides on MHC class I molecules (intrinsic signal 1). Blockade of PD-1 or PD-L1 enhances T cell activation.
REGN5678 (PSMA×CD28) exhibits dose-dependent antitumor activity when combined with cemipimab (anti-PD-1). CD28-targeted tumor combination immunotherapy can transform "cold" tumors resistant to anti-PD-1 therapy into "hot" tumors responsive to anti-PD-1 therapy. Other TAA×CD28 bispecific antibodies, such as EGFR×CD28 (NCT04626635), MUC16×CD28 (NCT04590326), and B7H3×CD28 (NCT05585034), are currently being evaluated in combination with PD-1 blockade for the treatment of various solid tumors.
Trispecific antibodies/multispecific antibodies
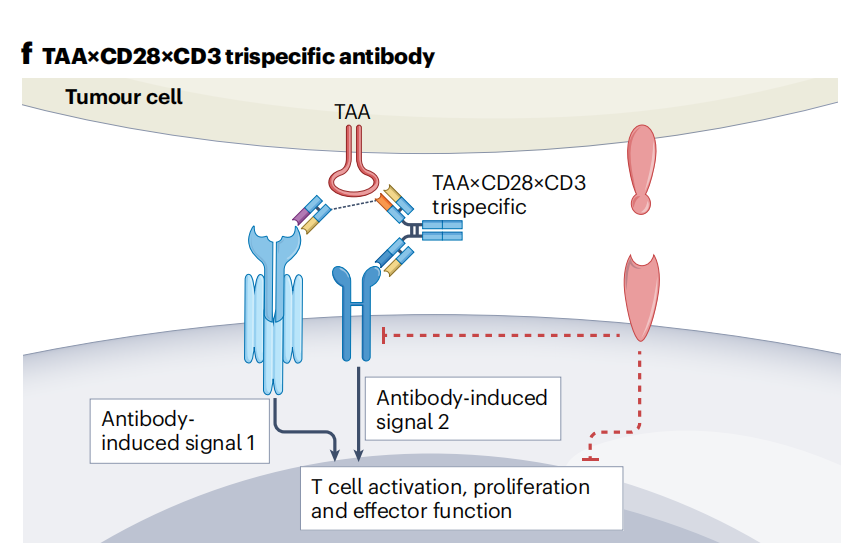
Other trispecific antibodies targeting various TAAs, CD3, and CD28 have been developed and clinically tested. Trispecific antibodies (TAA × CD28 × CD3) activate T cells by simultaneously delivering signal 1 and signal 2 to T cells near TAA-expressing tumor cells.
CC312 ( CD19 × CD3 × CD28 ) trispecific antibody is a single-chain antibody for the treatment of B-cell malignancies , and its safety, PK, PD, and immunogenicity are being studied in NCT06037018.
MDX2001 (TAA × TAA × CD3 × CD28) , a tetraspecific antibody designed to optimize T cell function while preventing tumor antigen escape by targeting two TAAs , has entered the clinic (NCT06239194 ) . It remains to be determined whether an antibody with fixed CD3- and CD28-binding geometries can mediate similar effects as a combination of two distinct bispecific antibodies (TAA × CD3 + TAA × CD28) administered separately.
Other forms of antibodies
Most of the antibodies currently in the clinic combine these signals by targeting PD-L1 on tumor cells and co-stimulatory receptors on immune cells in trans, as exemplified by bispecific antibodies targeting PD-L1 and CD28. Davoceticept (ALPN-202) was tested in combination with an anti -PD-1 agent in a phase 1 trial. Davoceticept , a CD80 extracellular domain fused to an effector-free IgG Fc, was designed to activate CD28 and bind to PD-L1. However, this trial was terminated due to cardiac deaths in two patients.

(Data source: Lotze MT, Olejniczak SH, Skokos D. Nat Rev Immunol. 2024)
