Endosialin, also known as tumor endothelial marker 1 (TEM1) or CD248, is a single-transmembrane glycoprotein with a C-type lectin-like domain. Endosialin is expressed in cancer-associated fibroblasts (CAFs) and pericytes in most tumors. Endosialin can promote tumor progression through various mechanisms, such as promoting tumor cell proliferation, adhesion, and migration, stimulating tumor angiogenesis, and inducing an immunosuppressive tumor microenvironment. Therefore, it is considered an ideal target for cancer therapy.
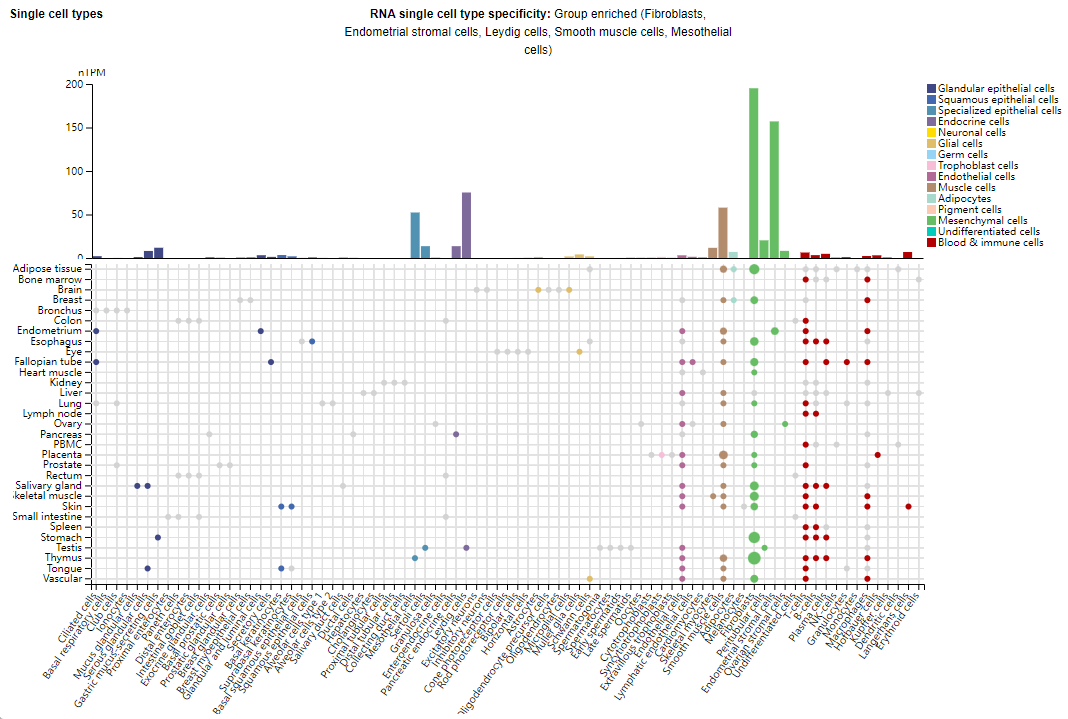
Expression distribution of CD248
CD248 is primarily expressed in fibroblasts, endometrial stromal cells, mesenchymal cells, smooth muscle cells, and mesothelial cells. In epithelial cancers, endothelin is primarily expressed in stromal cells, particularly fibroblasts and pericytes, while in mesenchymal sarcomas, endothelin is also expressed in tumor cells. Due to its specific high expression in various cancer types, endothelin is considered a promising therapeutic target for cancer treatment.
(Data source: Uniprot)
Structure of CD248
CD248 is a type I transmembrane glycoprotein belonging to the C-type lectin domain (CTLD) group 14 family . Endosomes are composed of a signal-leading peptide, five globular extracellular domains (including a C-type lectin domain, a domain similar to the Sushi/ccp/scr pattern and three EGF repeat sequences), a mucin-like region, a transmembrane segment and a short endoplasmic reticulum tail.
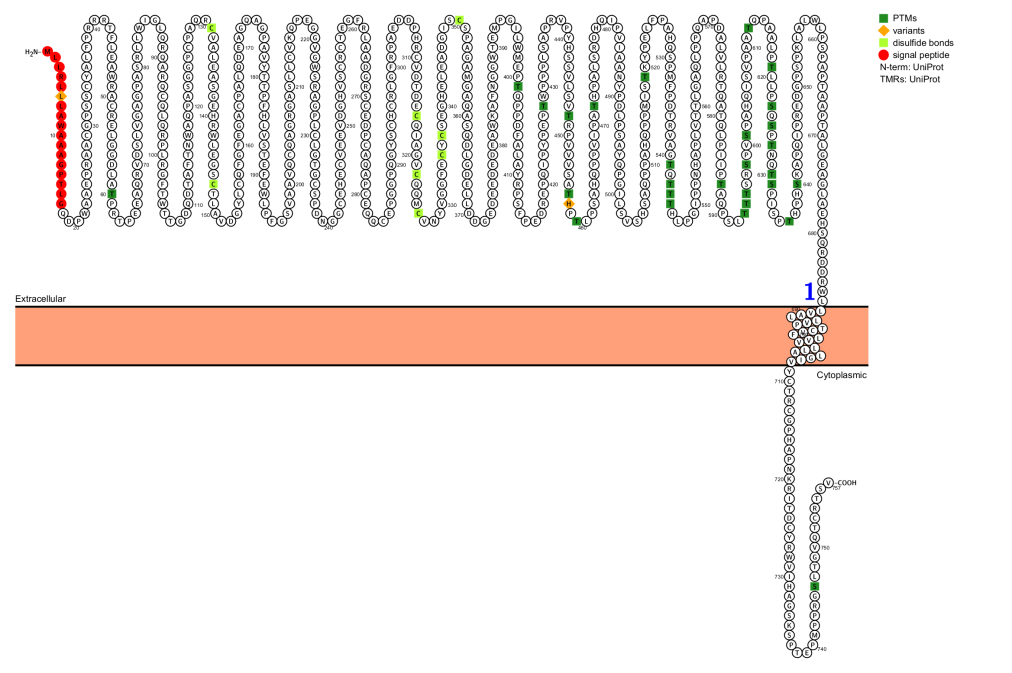
(Data source: uniprot)
The role of CD248 in promoting tumor progression
Endothelins are expressed in cancer cell stroma (CAFs) and pericytes, which are important components of the TME and contribute to the formation of an immunosuppressive TME, thereby promoting tumor progression.
CD48 promotes tumor proliferation, adhesion, and migration
In epithelial cell-derived tumors, endothelin may promote tumor cell proliferation, adhesion, and migration through cell-cell or cell-matrix interactions. Endothelin expressed by CAFs binds to Mac-2BP/90K expressed by tumor cells, promoting tumor cell adhesion and migration.
In mesenchymal sarcomas, endothelin expressed by tumor cells can bind to ECM proteins such as FN, Col I, and Col IV, enhancing cell adhesion and migration, or promote the interaction between ITGB1 and ECM proteins, activate the FAK-paxillin pathway, and promote the formation and migration of focal adhesions, thereby promoting tumor cell migration and invasion.

CD248 promotes tumor angiogenesis
CD248 is also involved in tumor angiogenesis. Endosialin is considered an effective target for anti-angiogenic therapy in various cancer types. Pericytes express endosialin , while endothelial cells express CLEC14A. CLEC14A binds to MMRN2 at the endothelial-pericyte interface, promoting tumor angiogenesis. Endosialin promotes pericyte proliferation by activating the ERK1/2/c-Fos pathway. Endosialin activates the Wnt/β-catenin signaling pathway in pericytes, upregulating two pro-angiogenic factors, OPN and SERPINE1, thereby promoting tumor angiogenesis.

CD248 induces an immunosuppressive tumor microenvironment
CD248 can induce an immunosuppressive TME, and its mechanism may be related to regulating the infiltration and exhaustion of CD8+ T cells or the recruitment and polarization of M2 macrophages .

(Data source: Lu S, et al. Theranostics. 2024)
CD248-targeted therapy
Due to its tumor-promoting function, CD248 is considered a promising anti-tumor target for endothelin-positive sarcomas and a variety of tumors with endothelin-positive stroma or blood vessels. Several antibodies specifically targeting CD248 have been developed , and different strategies targeting endothelin have been designed.
MORAb-004 ( Ontuxizumab ) , a humanized version of the mouse anti-human endothelin Fb5 antibody, is the first cancer therapeutic antibody to enter clinical trials . Because it lacks effective anti-tumor effects when used alone, researchers are pursuing other endothelin-targeting therapeutic strategies, such as radioimmunotherapy, ADCs (antibody-drug conjugates), CAR-T (chimeric antigen receptor T cells), BiTEs (bispecific T cell engagers), immunotoxins, immunoliposomes, nanoparticles, and even DNA vaccines.

hMP-E-8.3 is an antibody-drug conjugate that has demonstrated potent, specific, and target-dependent killing activity in vitro and achieved durable tumor growth inhibition in a cell line-based human osteosarcoma model. Endothelin is an attractive therapeutic target for osteosarcoma, and ADCs targeting endothelin have the potential to be developed as biotherapeutic drugs for these malignancies.
Fierle et al. constructed two types of CAR-T cells and soluble bispecific trivalent adapters (TriloBiTEs (tBs)) using two endothelin scFvs (1C1m and 7G22). They demonstrated that both types of CAR-T cells could specifically recognize and activate endothelin-positive target cells; the tBs could direct T cells to endothelin-positive target cells, and systemic delivery of 1C1m-tB could effectively prevent the establishment of Ewing sarcoma tumors in a xenograft model. These data further confirm that endothelin is a promising target for T cell-mediated immunotherapy.

(Data source: Lu S, et al. Theranostics. 2024)
